|
| Topics on Continuous Training |
A. Ortigado Matamala
Head of the Pediatric Service. Pediatric Cardiology. Guadalajara University Hospital. Professor of Medicine at the University of Alcalá
| Abstract
Syncope is defined as the sudden and self-limited loss of consciousness and postural tone resulting from a transient global cerebral hypoperfusion, sometimes preceded by a prodrome and always followed by a spontaneous complete recovery. Syncope is a common pediatric problem, although syncope is almost always benign, in a few cases it may be a clue to the presence of an underlying cardiovascular problem and may predict a risk of sudden death. A detailed history of the event, a comprehensive physical examination and an electrocardiogram will help in the differential diagnosis and must show the key features for identifying high-risk patients and exclude life threatening disorder. |
| Resumen
El síncope se define como la pérdida súbita y transitoria de la conciencia y del tono postural por una hipoperfusión cerebral transitoria, alguna vez, precedida por unos pródromos y, siempre, seguida de una recuperación completa y espontánea. El síncope es un problema pediátrico frecuente, afecta al 15-25% de los niños y, especialmente a los adolescentes, con un pico de incidencia entre los 15 y 19 años de edad, y con predominio en el sexo femenino. La etiología es variada, con múltiples causas y, aunque casi siempre es benigno, en unos pocos casos puede ser el aviso de una enfermedad cardiovascular subyacente con riesgo de muerte súbita. Una historia detallada del evento, una exploración física y un electrocardiograma, deben orientarnos en el diagnóstico diferencial y pueden mostrarnos los puntos clave para identificar pacientes de alto riesgo y enfermedades que amenacen la vida. |
Key words: Syncope; Vasovagal syncope; Cardiac syncope; Transient loss of consciousness; Sudden death; Pediatrics.
Palabras clave: Síncope; Síncope vasovagal; Síncope cardiaco; Pérdida transitoria del conocimiento; Muerte súbita; Pediatría.
Pediatr Integral 2021; XXV (8): 339 – 405
Syncope
Introduction
Syncope is the sudden loss of consciousness and postural tone, of brief duration and with spontaneous recovery.
Syncope is a sudden loss of consciousness and postural tone, due to a transient cerebral hypoperfusion, characterized by a rapid onset, a fleeting duration and a spontaneous and complete recovery(1). Syncope is a prevalent pathology in pediatric emergencies, generally benign, but generating great concern to the patient and his family. One of the challenges that syncope poses for the pediatrician is to identify the cases with underlying and potentially fatal cardiac pathology(2).
When we evaluate episodes of this nature, we must avoid terms such as: “dizziness”, “fainting”, “attack” or “crisis”, because they are ambiguous. We should also know how to differentiate a syncope from vertigo or balance disturbances, characteristic of cerebellar or vestibular dysfunction.
We must also know how to differentiate syncope from other situations with transient loss of consciousness (TLoC) which are not due to a transient cerebral hypoperfusion, such as: neurological causes (epilepsy, migraine, head trauma, transient cerebrovascular accident…), metabolic causes (hypoglycemia), poisonings (medications, drugs of abuse, carbon monoxide…) or those of psychogenic origin(3,4).
Although it seems a clear and well-defined topic, there are still open issues in the medical literature that raise the need for a consensus. There are two important international clinical practice guidelines for the diagnosis and management of syncope; on the one hand, the 2017 American guideline of the “American College of Cardiology/American Heart Association/Heart Rhythm Society” (ACC/AHA/HRS), and on the other hand, the 2018 fourth edition of the European guideline “European Society of Cardiology/European Heart Rhythm Association” (ESC/EHRA(5,6). Interestingly, there are certain differences between the American and the European guidelines. Another issue to highlight is that, although both guidelines are very thorough, both hardly expound on the subject of syncope in the pediatric age, where there are specific forms, such as sobbing spasms, with its two variants, pale and cyanotic.
It is also important not to confuse syncope with presyncope. The term “presyncope” refers to the clinical situation with the symptoms previous to syncope, that is, the vegetative symptoms including: dizziness, paleness, cold sweating and blurred vision, but without losing consciousness. Therefore, presyncope would be a failed episode of syncope.
If the cerebral anoxia in a syncope extends for more than 15 seconds, a convulsive syncope may occur, characterized by generalized tonic spasms, mandibular trismus, opisthotonos, myoclonic jerks and sphincter relaxation(7). Convulsive syncope, although infrequent (5% of syncope episodes), can pose one of the main challenges for the pediatrician in its differential diagnosis with an epileptic disorder(3,4).
Epidemiology
Syncope is common in pediatrics, especially in adolescence, being benign in most cases.
Syncope is a frequent clinical situation in Pediatrics, affecting 15-25% of children and adolescents, with a peak of maximum incidence at 18 years of age (30-50%). Syncope is more common in females, especially between the ages of 15 and 19 years(4). Syncope is uncommon below 6 years of age, except if there is an underlying neurological (epilepsy) or cardiac pathology (arrhythmia), or sobbing spasms. Syncope is the reason for attending the pediatric emergency service in 0.5-3 cases per 1,000.
Most episodes of syncope in pediatrics are benign, 75% correspond to reflex or neurally mediated syncope, where among them, vasovagal syncope, also known as neurocardiogenic syncope, stands out(10,11). Cardiac syncope accounts for 5-10%, and its prognosis underscores its great clinical interest. Recurrence rate varies between 33-51% in patients who have been followed for 5 years(9).
Etiopathogenesis
Syncope can have a varied etiology, where vasovagal syncope is the most common, however cardiac syncope can represent a potentially fatal underlying disease.
The causes of syncope are multiple, with various underlying pathogenic mechanisms, and not all of them completely clarified.
Low blood pressure and cerebral hypoperfusion are the main causes of syncope. Systemic blood pressure is determined by cardiac output and peripheral vascular resistance, and a decline in one of these two factors can cause syncope.
There are three causes of low vascular resistance:
1. Reflex response abnormality with vasodilation (vasodepressor-type reflex syncope).
2. Functional failure of the autonomic nervous system.
3. Structural failure of the autonomic nervous system.
Autonomic nervous system failure can be primary or secondary, for instance, drug-induced.
The four causes of low cardiac output are:
1. Reflex bradycardia (cardioinhibitory-type reflex syncope).
2. Structural heart diseases and arrhythmias, especially ventricular tachycardias.
3. Inadequate venous return: hypovolemia or venous accumulation.
4. Chronotropism and inotropism anomaly due to autonomic nervous system dysfunction.
It must be borne in mind that these different primary mechanisms can interfere and interact with each other(5).
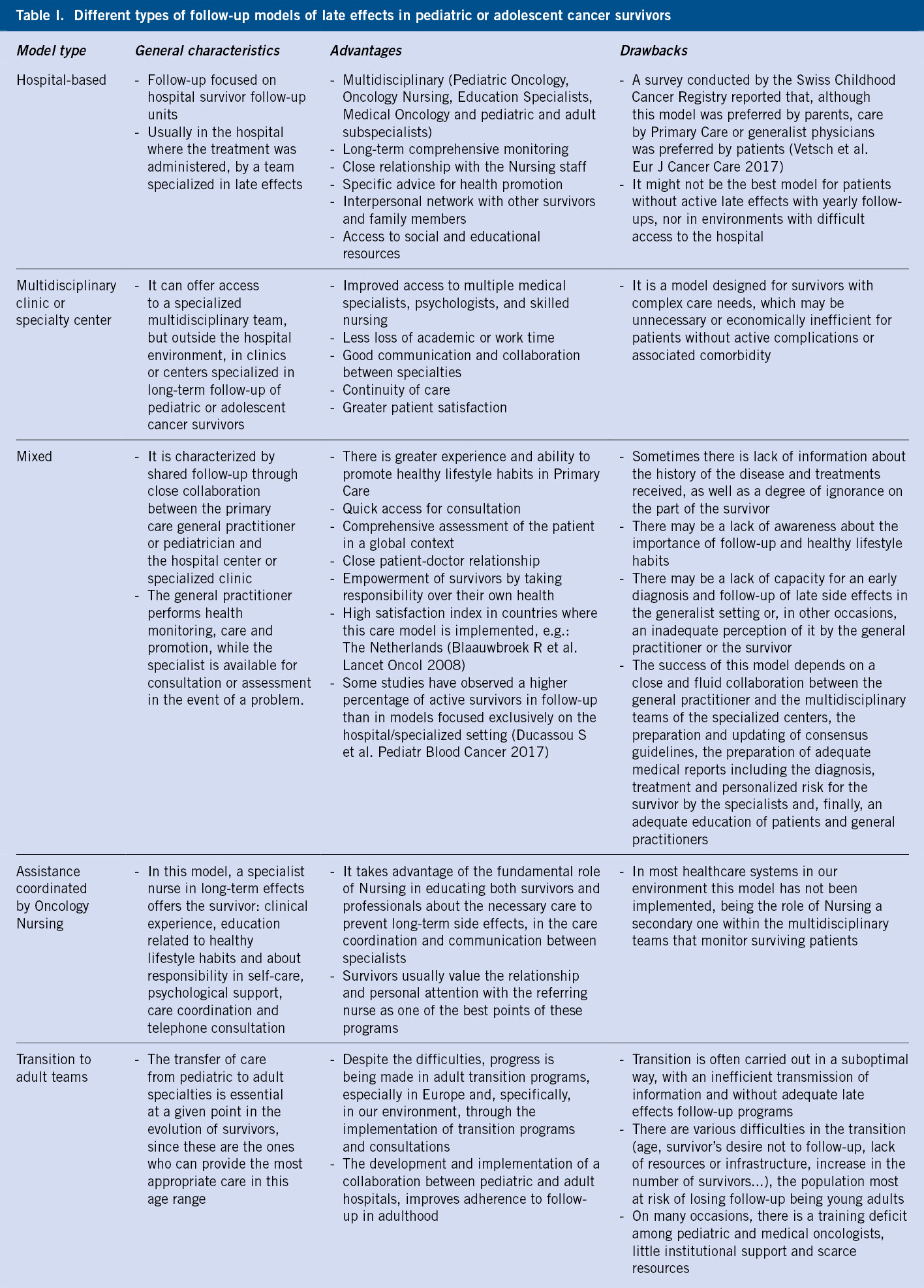
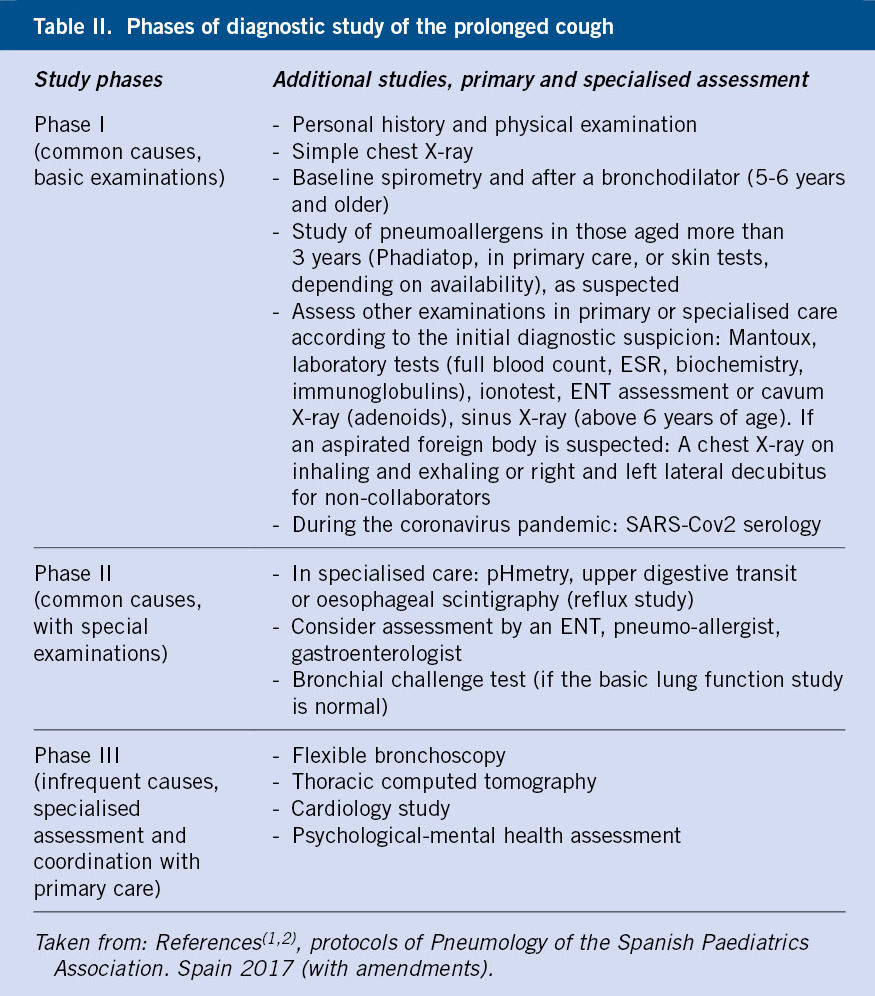
To facilitate the understanding and clinical management of syncope, it is advisable to classify syncope based on its etiopathogenesis, which can be summarized into 3 large groups (Table I):
1. Reflex or neurally mediated syncope.
2. Cardiac syncope
3. Non-cardiac syncope.
Reflex or neurally mediated syncope
Reflex syncope is the most common one (75%). Diverse factors such as erect position of the body and venous pool in the lower extremities condition a sudden decrease in cardiac preload, which for instance is accentuated in prolonged standing. There is a paradoxical reflex response in syncope. The relative hypovolemia due to a decrease in blood pressure causes an initial release of catecholamines to improve chronotropism and inotropism. However, cardiac contractions in relatively empty ventricles stimulate the left ventricular mechanoreceptors and via the afferent vagal pathway (unmyelinated C fibers) reach the brainstem. Subsequently, the efferent vagal response is twofold, causing bradycardia (cardioinhibitory response) and vasodilation (vasodepressor response).
Nevertheless, the mechanisms involved are broader and require further clarification. Syncope triggered by an intense emotion, the sight of blood or trauma from afar, suggest the involvement of brain structures. There are studies that indicate the existence of low levels of serotonin and high levels of beta-endorphins in patients with this type of syncope(10).
Another relevant component that has been related to neurally mediated syncope is endothelial function(11,12). Nitric oxide (NO) is a powerful vasodilator in our body, generated by endothelial cells through the nitric oxide synthase (NOS) enzyme. In children and adolescents with this type of syncope, higher levels of NO and NOS have been detected than in the control group(13).
Vagal or neurocardiogenic syncope is the most frequent and representative form of this type of syncope, where, in addition to the mechanisms already described, there is usually a precipitating factor that would act as a trigger, such as: emotional distress (anxiety, fear, pain) or physical stress (heat, prolonged standing, fatigue, dehydration, fasting, intercurrent illness…).
Sometimes syncope occurs in specific circumstances, such as during urination, especially in men (whilst standing) and in the first void of the morning (fasting and after sitting up from a previous lying down position). Other situational syncope episodes are related to defecation, ingestion of cold drinks, physical exertion, hot baths, coughing, intense laughter, blowing or playing wind instruments (Valsalva maneuver).
Another clinical picture within this group is carotid sinus syndrome. The carotid sinus is a regulator of blood pressure through its baroreceptors. In case of hypersensitivity, performing a stimulus such as a carotid sinus massage can lead to syncope(14).
In Pediatrics, there is a specific entity of reflex syncope called sobbing spasms with its two variants, the cyanotic and the pale type. Cyanotic-type sobbing spasm occurs between 6 months and 5 years of age, peaking at 2 years, in which the syncope begins with a loud cry (“tantrum”) followed by an apnea pause. The pale-type sobbing spasm appears between 12 and 24 months of age, and the syncope occurs suddenly after a stimulus (pain or fright) and without prior crying. The prognosis of these clinical entities is good, 100% will disappear with age. 25% of the cases of pale-type sobbing spasms will develop vasovagal syncope in adolescence(3).
Another entity to take into account is postural orthostatic tachycardia syndrome (POTS), frequent in adolescents, especially in females. This entity consists of an intolerance to standing that occurs in the first 10 minutes after modifying the posture to standing up, with an acceleration of the heart rate greater than 30 bpm (maximum rate of: 130 bpm for 6-12 years, 125 bpm for 13-18 years, and more than 120 bpm in adults) in the absence of hypotension. Dizziness, weakness, blurred vision, and syncope may manifest along with tachycardia. POTS would be an exaggeration of a compensatory physiological mechanism. Although its pathophysiology is not fully clear, it has been related to various factors such as: hypovolemia, iron deficiency, hyperadrenergic state, dysfunction of local regulation of vascular tension, endothelial dysfunction, autoimmune dysfunction (antibodies against cholinesterase receptors) or mast cell activation(15).
Cardiac syncope
Although syncope of cardiac origin is rare (5-10%), it is of great significance due to its morbidity and mortality. Syncope in these cases may be the first manifestation of a life-threatening heart disease(16).
Globally, they can be classified into three large groups: the first one being situations of obstruction to the ventricular outflow tract; in second place, myocardial dysfunction and, finally, arrhythmias.
Left ventricular outflow tract obstructions include aortic stenosis and obstructive hypertrophic cardiomyopathy, whereas in the case of the right ventricle, severe pulmonary stenosis and primary pulmonary hypertension stand out(3).
Myocardial dysfunction can be primary (e.g., familial cardiomyopathies) or secondary (e.g., viral myocarditis). Ischemic cardiomyopathy due to coronary lesion is uncommon in Pediatrics, but, as a congenital pathology, we must bear in mind the Anomalous origin of the Left Coronary Artery from the Pulmonary Artery (ALCAPA) syndrome and, as an acquired pathology, Kawasaki disease(17).
Among the bradyarrhythmia that can cause syncope, carotid sinus disease is an etiological condition, and, above all, complete atrioventricular block, which can be congenital (child of mother with systemic lupus erythematosus) or acquired (post-cardiac surgery, inflammatory/infectious diseases of the heart)(4).
Tachyarrhythmias are also a cause of syncope. Supraventricular tachycardias (e.g., Wolff-Parkinson-White syndrome) are usually better tolerated in Pediatrics, but ventricular tachycardias have a worse prognosis, being potentially fatal. Special mention should be made to channelopathies, such as long QT syndrome, short QT syndrome, Brugada syndrome or catecholaminergic polymorphic ventricular tachycardia that, having structurally normal hearts, are associated with severe ventricular tachyarrhythmias that can culminate in sudden death. Physicians must recall that the QT interval can be lengthened, not only congenitally (17 subtypes of long QT syndrome have already been described), but also acquired by electrolyte disturbances (hypokalemia) and especially by the administration of drugs (certain antiarrhythmics, psychotropics, macrolides or antihistamines)(18). It is important to be aware of these medications, especially in patients with congenital long QT syndrome. The lists of drugs that prolong the QT interval, grouped into three categories according to the level of evidence, are available on the website of the Arizona Center for Education and Research in Therapeutics (AZCERT) (www.crediblemeds.org). These lists are periodically updated taking into consideration notifications from regulatory agencies and available clinical evidence. One only has to register for free on the website to receive these updates(19).
Non-cardiac syncope
There are other entities that, although they do not meet the requirements of syncope, must be taken into consideration, especially for the differential diagnosis with other forms of transient loss of consciousness (TLoC).
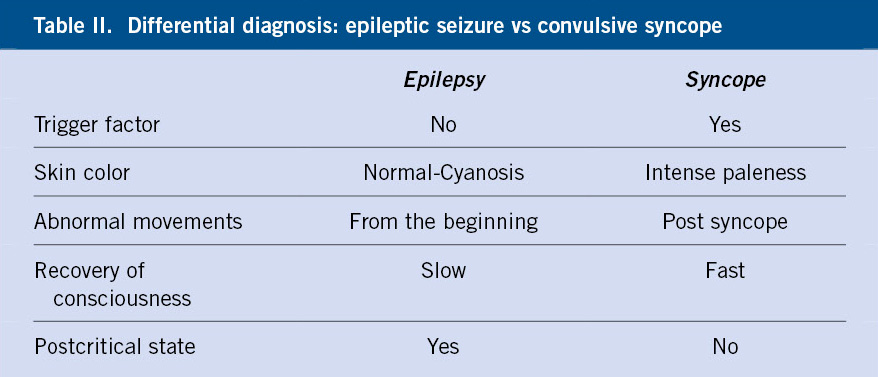
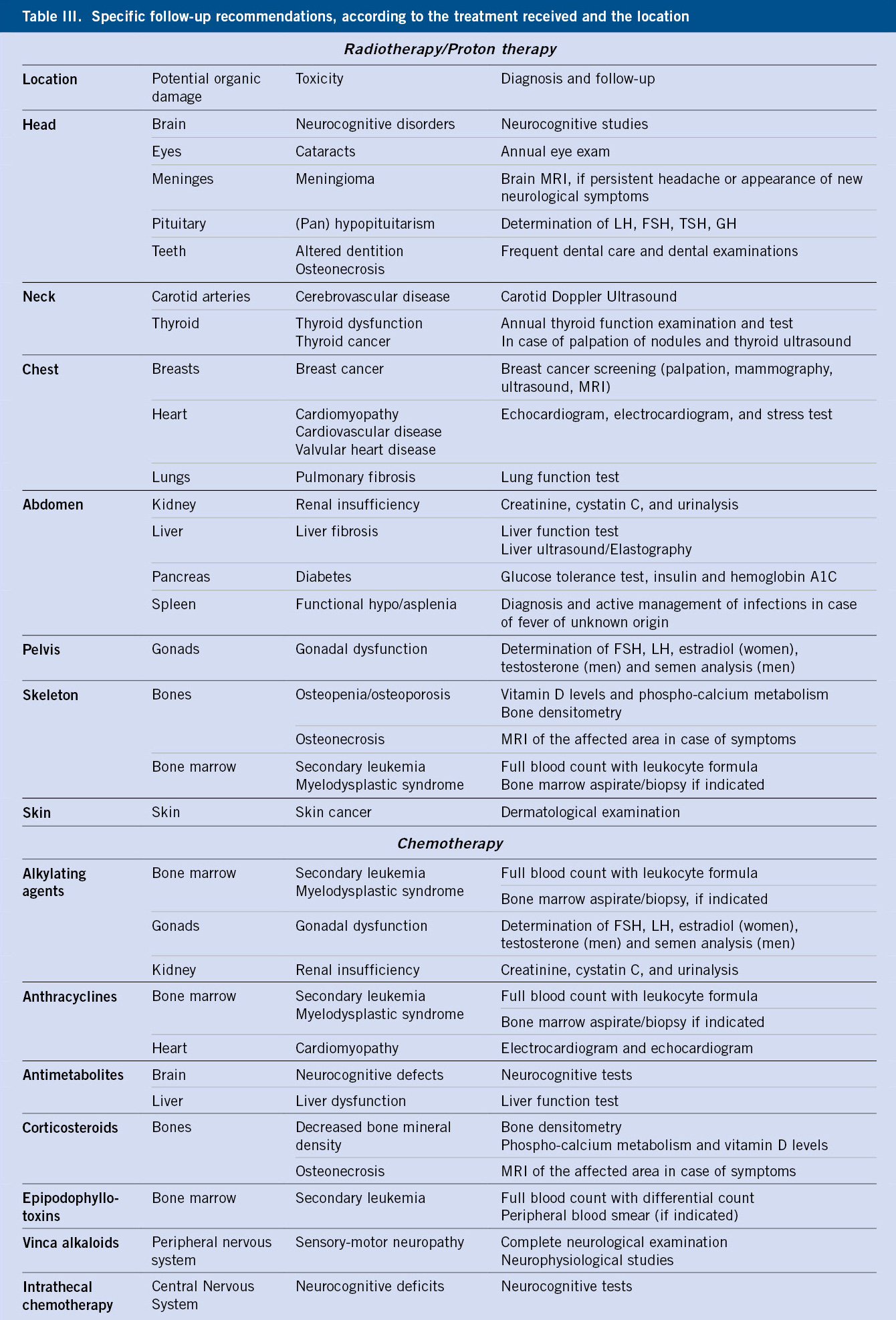
In this group, epilepsy should be highlighted, due to the challenge it poses for the physician so as not to confuse an epileptic seizure with a syncopal seizure (Table II).
Other neurological abnormalities that can simulate a syncopal episode are migraines (basilar migraine), transient cerebrovascular accidents, cataplegic seizures in narcolepsy, “vestibular syncope” (Tumarkin otolithic seizures or drop attacks) or acute elevations of cerebrospinal fluid pressure (hydrocephalus)(3).
Among the “psychogenic syncope” entities, various disorders of a psychiatric nature must be taken into account, such as hysterical crises, panic attacks, conversion reactions or major depression. In case of hyperventilation, hypocapnia occurs which, if not compensated, derives in acute respiratory alkalosis, with dizziness, confusion, peripheral and perioral paresthesias, cramps and finally syncope(4).
Hypoglycemia can mimic syncopal manifestations due to the adrenergic response associated with: anxiety, tachycardia, sweating, paleness, tremor, weakness, and sleepiness.
We should not forget the possibility of ingestion of toxins, medications or drugs of abuse in patients with syncope, especially in adolescents. Among medications, there are those that lengthen the QT segment of the electrocardiogram, drugs that decrease blood volume (antihypertensives and diuretics), and psychotropic drugs(10).
Diagnosis
The diagnosis of syncope is based on a detailed medical history, a full physical examination, and an electrocardiogram.
The diagnosis of syncope is based on three basic pillars:
1. Medical history.
2. Physical examination.
3. Electrocardiogram (EKG).
Medical history
The medical history is the cornerstone and essential tool in the diagnosis of syncope, with a detailed recall of all the information, both from the patient and from possible witnesses. The clinical history must include the following information(5):
• Personal and family history. It is important to know the patient’s history and possible diseases of interest (cardiac diseases, epilepsy, diabetes…), as well as family´s (cardiomyopathies, cardiac arrhythmias, cases of sudden death, vasovagal syncope in parents…). In adolescent girls, assess the possibility of pregnancy.
• Predisposing factors. Look for data on the circumstances prior to syncope, which may be predisposing factors, such as: possible fasting, medication intake, the environment where it took place (indoor or outdoor premises, temperature, crowded spaces), activity carried out (rest, sport, bath, urination…) or position of the patient (standing, decubitus or sitting).
• Triggering or precipitating factors. The report of the episode should consider possible syncope triggers that may have acted as a trigger for the loss of consciousness (postural change, panic, fear, trauma…).
• In congenital long QT syndromes, syncope may be associated with severe ventricular arrhythmia (“Torsade de pointes”) induced by a specific trigger. Type 1 (LQTS1) is related to physical exercise (especially swimming), type 2 (LQTS2) to emotional stress or a sudden intense auditory stimulus, and type 3 (LQTS3) with sleep (bradycardia).
• Initial clinical manifestations (prodrome). Next, the initial symptoms that precede the loss of consciousness (paleness, sweating, nausea, vomiting, abdominal pain, chest pain, palpitations, weakness, dizziness, blurred vision…) should be described.
• Description of the syncope. The characteristics of the episode of transient loss of consciousness (TLoC), its duration, body attitude, mucocutaneous color and description of possible abnormal movements (convulsive crisis?) are important.
• Later recovery. Confirm normality, later symptoms or existence of a post-critical state (drowsiness, confusion, neurological focus…).
Physical examination
The physical examination should be complete, paying special attention to cardiovascular signs (palpation of central arterial pulses, heart rate or auscultation of heart murmurs) and neurological signs (level of consciousness, balance, vestibular function, neurological focus…). Vital signs should be measured, especially heart rate and blood pressure.
An important issue to assess in the physical examination is changes with orthostatism, requesting the patient to move from the decubitus position to standing and after 3 minutes checking if symptoms appear and measuring the heart rate and blood pressure. A drop of more than 20 mmHg in systolic blood pressure, or 10 mmHg in diastolic blood pressure, or an acceleration in the heart rate greater than 30 bpm, suggests a neurally mediated mechanism of the syncope(4).
One method to evaluate autonomic function is through the Valsalva maneuver, which is more profitable in adult patients than in Pediatrics. The patient performs a forced expiration for 15 seconds with the glottis closed (mouth and nose closed). Initially, blood pressure rises slightly due to filling of the left ventricle, but soon decreases due to the abrupt reduction in venous return, cardiac output decreases, and heart rate increases to compensate (baroreceptor reflex). This hypotension in healthy individuals will cause a compensatory automatic sympathetic response: it increases systemic vascular resistance and heart rate. At the end of the maneuver, when the patient releases all the trapped air and breathes normally, the intrathoracic overpressure disappears, a drop in blood pressure occurs, and the heart rate normalizes. In situational syndrome, for example, when playing a wind instrument or lifting excessive weight, normal heart rate response is associated with pronounced hypotension due to lack of the compensatory automatic response(6).
Another of the diagnostic tests is the carotid sinus massage, which reproduces the syncope with a reflex mechanism (bradycardia and hypotension), however, this maneuver is not indicated in Pediatrics.
Complementary tests
In addition to measuring blood pressure in decubitus and standing position, a capillary blood glucose should also be checked.
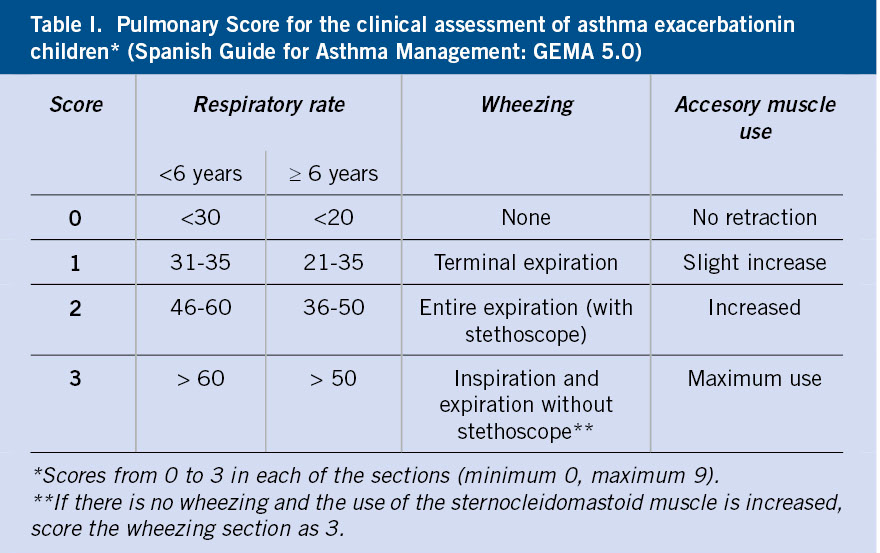
There is a broad consensus on the recommendation to perform a 12-lead electrocardiogram (EKG) in all syncope episodes, although the Canadian guidelines indicate it in an atypical syndrome or if associated risk factors(7,8). In the EKG the heart rate and rhythm should be checked, arrhythmias should be ruled out, and the presence of sinus rhythm confirmed.
Even in an EKG with sinus rhythm, the following warning signs, indicative of possible cardiac pathology in syncope, should be ruled out:
• Wolff-Parkinson-White (WPW) syndrome pattern: short PR interval (from the beginning of the P wave to the beginning of the QRS complex), wide QRS complex and delta wave (initial filling of the QRS complex, positive or negative).
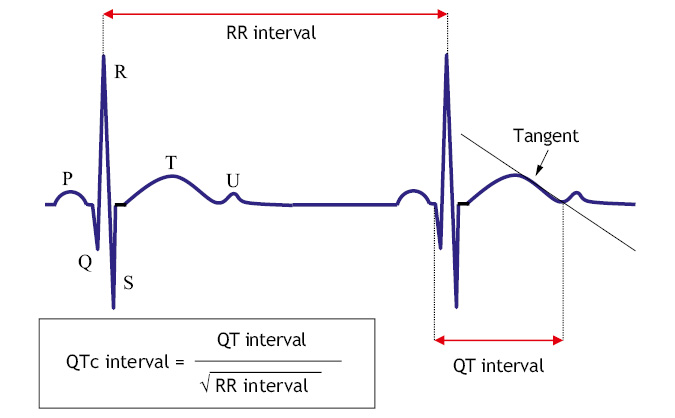
• QT interval: the corrected QT (QTc) must be obtained using the Bazett formula, because the QT interval varies with heart rate (Fig. 1), preferably measured in leads II and V5. Remember that the QT interval goes from the beginning of the Q wave to the end of the T wave (that of the tangent of the T wave), not including the U wave, if identified.
Figure 1. Calculation of the QTc (Bazzet’s formula).
• Long QT syndrome (LQTS): QTc prolongation, greater than 450 msec in men and greater than 460 msec in women.
• Short QT syndrome (SQTC): shortening of the QTc, less than 330 msec.
• Brugada syndrome pattern: downward elevation of the ST segment greater than or equal to 2 mm (“shark fin”) in more than one right lead (V1-V2), followed by negative T waves (type I pattern).
• Hypertrophic cardiomyopathy: left ventricular enlargement with changes in repolarization, pathological wide and deep Q waves, unevenness of the ST segment, negative T waves on the lower and left side.
• Arrhythmogenic cardiomyopathy: presence of epsilon waves which, although infrequent, are specific (electrical potentials of low amplitude between the end of the QRS complex and the beginning of the ST segment), inverted T waves in the right precordial (V1, V2, V3) in individuals older than 14 years of age and in the absence of complete right bundle branch block.
The tilt-table test or upright tilt testing allows the provocation of syncope symptoms in predisposed people and under a controlled situation with monitoring of blood pressure, heart rate, respiratory rate and peripheral oxygen saturation. Tilt table performance is suboptimal. The indication for this test would be limited to patients with recurrent syncope and reflex syncope suspicion, but in whom the clinical evaluation has failed to specify it. Therefore, the tilt table test is useful for the diagnosis of reflex syncope, in autonomic failure that occurs with orthostatic hypotension, in postural orthostatic tachycardia syndrome (POTS), and it could also be useful in differentiating a vasovagal syncope from a psychiatric pseudosyncope(6).
Electroencephalogram (EEG) is not indicated as a routine test in syncope and would be limited to those cases with suspected underlying neurological etiology, specifically, epilepsy. An epileptic seizure with loss of consciousness can simulate a convulsive syncope and, therefore, it is important to consider it in the differential diagnosis (Table II). Neuroimaging tests, such as Computerized Axial Tomography (CT) or Magnetic Resonance Imaging (MRI), would also be limited in their indication, for the same reason.
Blood tests, except for capillary blood glucose, are not necessary in every syncope, hence full blood count (e.g., hypovolemia or infection) or biochemistry (suspected metabolic abnormality) must be taken only if justified by clinical criteria. In certain cases, the possibility of requesting urine toxicology tests or a pregnancy test should be taken into account.
If the patient is referred to the pediatric cardiology clinic, the study can be completed with echocardiography, ambulatory EKG-Holter and ergometry. In specific cases, other studies may also be indicated such as: cardiac MRI, subcutaneous implantable Holter, electrophysiological study and cardiac catheterization.
Treatment
Treatment is based on three main pillars: reassuring, educating and preventing.
Most syncope episodes are neurally mediated and it is, therefore, important to inform the patient and family about the benign nature of the process, avoiding or minimizing predisposing and triggering factors for syncope episodes (avoidance of prolonged standing, stressful, hot environments or agglomerations). Thus, it is important to help them to learn how to detect prodromal symptoms and guide them with instructions to carry out maneuvers to interrupt the episode (supine position, physical counter-pressure maneuvers)(20).
The most common counterpressure maneuvers are the following three(6):
1. In a standing position cross the legs, making maximum tension of the muscles of the legs, abdomen and buttocks, in an intense and sustained manner.
2. Contraction of hands, squeezing a rubber ball or a soft object with the greatest possible force, in an intense and sustained way.
3. Tense the arms, with the hands remaining intertwined and correctly grasped, producing an intense and sustained isometric contraction, with the arms in the horizontal plane and pulling outwards with the elbows (centrifugal force).
As a general rule, maintaining good hydration (fluid intake 30-50 mL/kg/day, 1.5-2.5 L/day) and a controlled increase in salt intake (2-5 g/day ) are recommended(7).
The use of pharmacological treatment is controversial and limited to specific cases (fludrocortisone, beta-blockers, alpha1-agonists).
Role of the Primary Care pediatrician
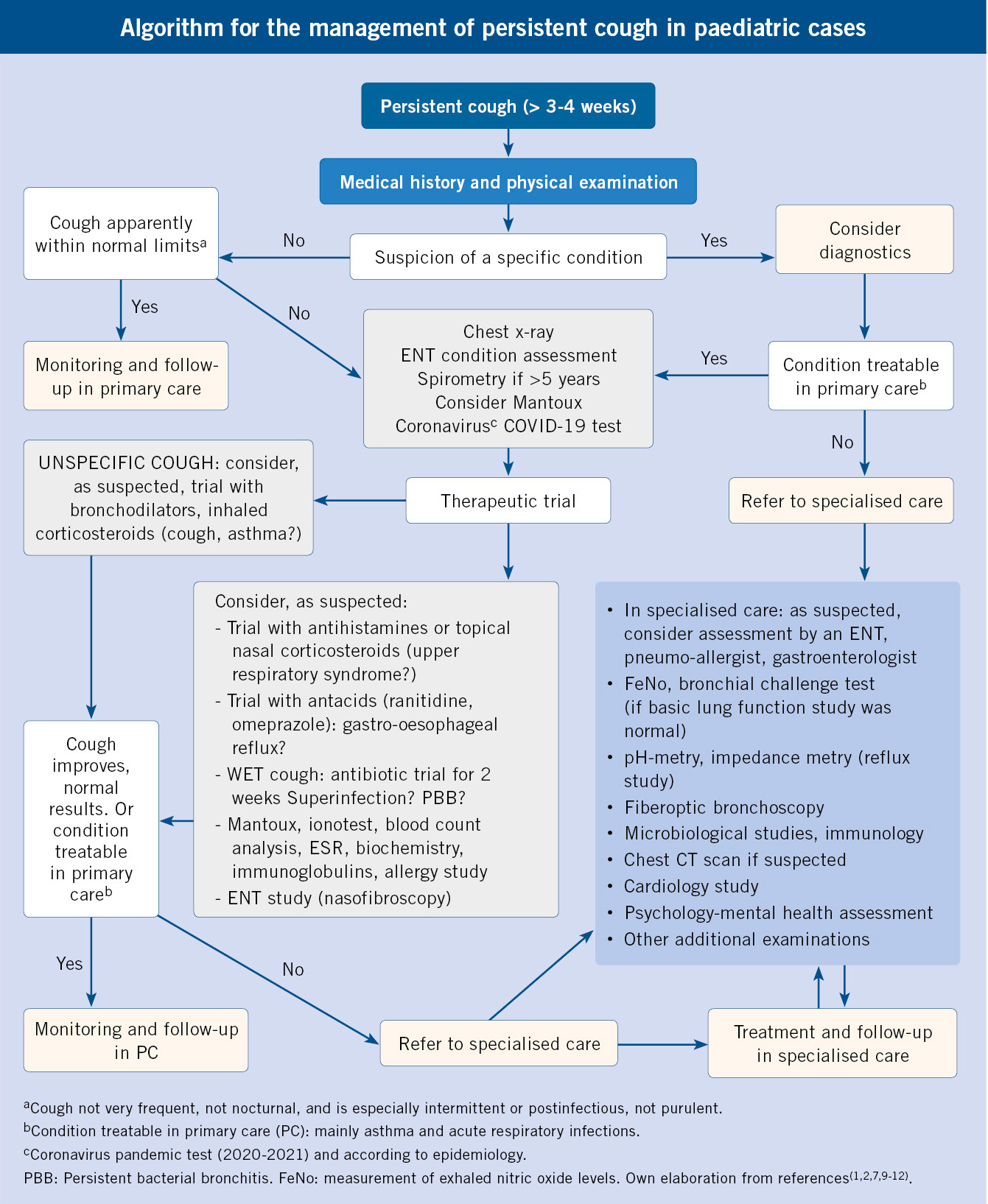
The Primary Care pediatrician is essential in syncope, because it is usually the doctor responsible for its initial assessment and the one who decides its clinical management.
Syncope is a frequent cause of consultation in Primary Care and, the pediatrician, the doctor who attends it. Most syncope episodes are benign, and a detailed medical history, a complete physical examination, and an EKG are sufficient to reach a diagnosis. This is the case of neurally mediated syncope, specifically, vasovagal syncope, which does not require further study or referral to a specialist, but does need to inform the patient and family of the benign nature of the condition, and of its possible prevention or how to act in the event of another episode(21).
One of the challenges for the pediatrician in a patient with syncope is to detect the possibility of an underlying pathology, especially of cardiac origin, which requires referral to the hospital emergency department or to the cardiology clinic (Fig. 2).
Figure 2. Syncope clinical management algorithm. PAT: pediatric assessment triangle.
Bibliography
The asterisks show the interest of the article in the opinion of the author.
1.** Zavala R, Metais B, Tuckfield L, DelVecchio M, Aronoff S. Pediatric syncope: a systematic review. Pediatr Emer Care. 2020; 36: 442-5.
2.*** Koene RJ, Adkinsson WO, Benditt DG. Syncope ant the risk of sudden cardiac death: evaluation, management and prevention. J Arrhythm. 2017; 5: 33-44.
3.*** Kanjwal K, Calkins H. Syncope in children and adolescents. Cardiol Clin. 2015; 33: 397409.
4.** Anderson JB, Willis M, Lancaster H, Leonard K, Thomas C. The evaluation and management of pediatric Syncope. Pediatr Neurol. 2016; 55: 6-13.
5.** Shen W-K, Sheldon RS, Benditt DG, Cohen MI, Forman DE, Goldberger ZD, et al. 2017 ACC/AHA/HRS Guideline for the evaluation and management of patients with syncope. A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2017; 136: e60-122.
6.*** Brignole M, Moya A, de Lange FJ, Deharo JC, Elliott PM, Fanciulli A, et al. ESC Scientific Document Group. 2018 ESC Guidelines for the diagnosis and management of syncope. Eur Heart J. 2018; 39: 1883-948.
7.*** Moodley M. Clinical approach to syncope in children. Semin Pediatr Neurol. 2013; 20: 12-7.
8.*** Sanatani S, Chau V, Fournier A, Dixon A, Blondin R, Sheldon R. Canadian Cardiovascular Society and Canadian Pediatric Cardiology Association Position Statement on the Approach to Syncope in the Pediatric Patient. Can J Cardiol. 2017; 33: 189-198.
9.** Zhang Q, Du J, Wang Z, Du Z, Wang L, Tang C. The diagnostic protocol in children and adolescents with syncope: a multi-center prospective study. Acta Paediatr. 2009; 98: 879-84.
10.** Puñal JE, Rodríguez A, Gómez C, Martinón-Torres F, Castro-Gago M, Martinón JM. Síncope en el adolescente. Orientación diagnóstica y terapéutica. An Pediatr. 2005; 63: 330-9.
11.** Dehghan B, Sabri MR, Javanmard SH, Ahmadi AR, Mansourian M. Neurally mediated syncope: is it really an endothelial dysfunction? Anatol J Cardiol. 2016; 16: 701-6.
12.** Santini L, Capria A, Brusca V, Violo A, Smurra F, Scarf I. et al. An increased endothelial-independent vasodilation is the hall mark of the neurally mediated syncope. Clin Cardiol. 2012; 35: 107-10.
13.** Liao Y, Chen S, Liu X, Zhang Q, Ai Y, Wang Y, et al. Flow-mediated vasodilation and endothelium function in children with postural orthostatic tachycardia syndrome. Am J Cardiol. 2010; 106: 378-82.
14.** Moya-i-Mitjans A, Rivas-Gándana N, Sarrias-Mercè A, Pérez-Rodón J, Roca-Luque I. Síncope. Rev Esp Cardiol. 2012; 65: 755-65.
15.** Chen G, Du J, Jin J, Huang Y. Postural tachycardia syndrome in children and adolescents: pathologhysiology anc clinical management. Font Pediatr. 2020; 8: 34. Doi:10.3389/fped.2020.00474.
16.** Hammond BH, Zahka KG, aziz PF. Suden cardiac death: a pediatrician´s role. Pediatr Rev. 2019; 40: 456-67.
17.** Lee TM, Hsu DT, Kantor P, Towbin JA, Ware SM, Colan SD, et al. Pediatric cardiomyopathies. Circ Res. 2017; 121: 855-73.
18.** Wallace E, Howard L, Liu M, O´Brien T, Ward D, Shen S, et al. Long QT syndrome: genetics and future perspective. Pediatr Cardiol. 2019; 40: 1419-30.
19.** Web de Arizona Center for Education and Research on Therapeutics. Accessed september 6th 2021. Available at: www.azcert.org o www. qtdrugs.org.
20.** Strickberger SA, Benson W, Biaggioni I, Callans DJ, Cohen MI, Ellenbogen KA, et al. AHA/ACCF scientific statement on the evaluation of syncope. From the American Heart Association Councils on clinical cardiology, cardiovascular nursing, cardiovascular disease in the young, and stroke, and the quality of care and outcomes research interdisciplinary working group; and the American College of cardiology Foundation in collaboration with the Heart Rhythm Society. J Am Coll Cardiol. 2006; 47: 473-84.
21.** Rivera DR, Cartón AJ. Síncope. En: Guerrero-Fernández J, Cartón A, Barreda A, Menéndez J, Ruiz J, ed. Manuel de Diagnóstico y Terapéutica en Pediatría. Madrid: Editorial Médica Panamericana S.A.; 2018. p. 325-30.
Recommended bibliography
- Brignole M, Moya A, de Lange FJ, Deharo JC, Elliott PM, Fanciulli A, et al. ESC Scientific Document Group. 2018 ESC Guidelines for the diagnosis and management of syncope. Eur Heart J. 2018; 39: 1883-948.
Clinical practice international guide where all the scientific evidence on syncope is collected and evaluated. This article is important to clarify concepts and definitions, as well as to assist the physician in the evaluation, management, and treatment of these patients.
- Kanjwal K, Calkins H. Syncope in children and adolescents. Cardiol Clin. 2015; 33: 397409.
Article that focuses on syncope in the pediatric patient, describing its peculiarities and providing a global vision of the subject.
- Moodley M. Clinical approach to syncope in children. Semin Pediatr Neurol. 2013; 20: 12-7.
Short article in length, but helpful for the pediatrician by providing a practical approach in the clinical management of syncope in Pediatrics.
- Koene RJ, Adkinsson WO, Benditt DG. Syncope ant the risk of sudden cardiac death: evaluation, management and prevention.J Arrhythm. 2017; 5: 33-44.
This article highlights the importance of syncope as a clinical presentation of sudden death in different situations and diseases.
- Sanatani S, Chau V, Fournier A, Dixon A, Blondin R, Sheldon R. Canadian Cardiovascular Society and Canadian PediatricCardiology Association Position Statement on the Approach to Syncope in the Pediatric Patient. Can J Cardiol. 2017; 33: 189-198.
This article presents a very thorough guide of the approach to syncope and it is specific for pediatric patients.
|
Clinical case |
|
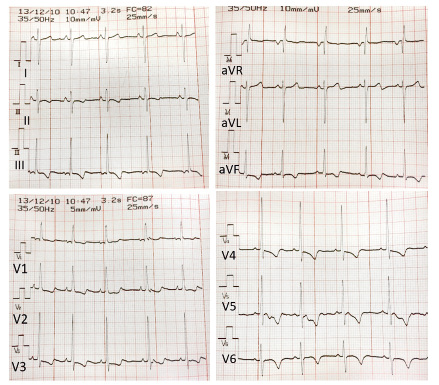
A school teacher observes and reports how a healthy 9-year-old boy, whilst playing with his friends in the schoolyard, right after standing up, suddenly loses consciousness, subsequently lying on the floor without abnormal movements. After a minute he regains consciousness, he is scared and only refers that prior to losing consciousness he noticed pain in his chest. His parents are notified and, although the child is apparently asymptomatic, they attend their pediatrician´s clinic at the Health Care Center. The pediatrician already knows the patient, as he is a thin child (weight in 3rd centile), with lack of appetite and gets tired easily. For this reason, his pediatrician had already requested a complete blood test a few weeks before. There are no data of interest in the personal and family history. On physical examination the patient is in good general condition, blood pressure 100/65 mmHg, oxygen saturation 98%, a new heart murmur is detected, a systolic murmur 2/6 in the tricuspid focus, the rest of the examination being normal. An EKG is performed (Fig. 3). Figure 3. 12-lead EKG. Note that the voltage setting in the frontal and precordial leads changes (10 mm/mV vs. 5 mm/mV). |