|
| Topics on Continuous Training |
J. Pellegrini Belinchón*, C. Ortega Casanueva**, S. de Arriba Méndez***
*Primary Care Pediatrician. Pizarrales Health Center. Salamanca. **Pediatrician and Allergist. Child Allergy and Pneumology Unit. Quironsalud San José Hospital. Madrid. ***Pediatrician specialized in Childhood Allergology and Pulmonology. Salamanca University Clinical Hospital
| Resumen
Asthma is the most common chronic disease among children.
|
Key words: Childhood asthma; Crisis; Treatment; Anti-asthma drugs.
Palabras clave: Asma infantil; Crisis; Tratamiento; Fármacos antiasmáticos.
Pediatr Integral 2021; XXV (2): 67 – 75 – EN
New approach in the treatment of children with asthma
Introduction
Asthma is one of the most prevalent chronic diseases in childhood(1).
According to the ISAAC study (International Study of Asthma and Allergies in Childhood), its prevalence in Spain is 10%, a similar number to that of the European Union, being more frequent in males aged 6-7 years(2).
Asthma constitutes a public health problem(3-5), entailing high social and health costs, as it is a frequent reason for consultation, both in pediatric emergency services and in the Primary Care setting.
The cost of asthma in Pediatrics in Spain depends on the severity of the disease. Blasco Bravo et al.(6) identified, a decade ago, that the total cost of asthma in Pediatrics in Spain was about 532 million euros, but it could range between 392 and 693 million euros. Direct costs (healthcare costs) represented 60% of the total cost whereas indirect costs (care provider’s time) 40%. A problem of this magnitude requires the optimization of resources against this disease and enhances the relevance of educating patients and their families.
In May 2020, the new update of the Spanish Guide for Asthma Management(7) (GEMA5.0) was published. Like the previous versions, it is a very practical, independent and consensual guide; but on this occasion it has been agreed on by twenty-one societies and scientific groups, among which is the Spanish Society of Primary Care Pediatrics (SEPEAP). This multidisciplinary guide allows a consensual approach and treatment of asthma, differentiating the management in those older or younger than 3-4 years of age, and it has become the main reference for the management of this disease among pediatricians in different areas of care.
The Global Initiative for asthma (GINA)(8), updated in 2020, is another essential guide for the management of asthma that provides treatment guidelines, according to the age of the child: those five years old or younger, and conversely, those aged six-years-old and above who will follow recommendations equal to those of adolescents and adults.
This manuscript will address: the evaluation and pharmacological treatment of exacerbations, the indications for maintenance treatment, the drugs used for this purpose, the treatment of exercise-induced bronchospasm and, finally, the education of the patient with asthma.
Assessment and treatment of asthma exacerbation
In a child with symptoms of asthma attack, once other diagnoses have been excluded, a rapid assessment of the severity of the exacerbation must be carried out to determine if immediate action is necessary and to apply treatment.
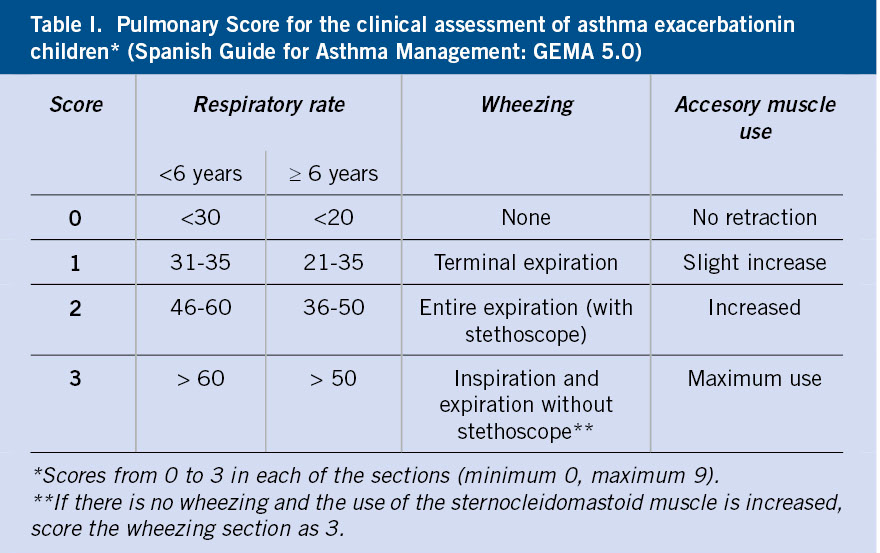
The severity assessment is mainly based on clinical criteria (respiratory rate, presence of wheezing and existence of retractions of the sternocleidomastoid muscle). Although no clinical scales are well validated(9,10), the Pulmonary Score (Table I) is simple and applicable to all ages(11).
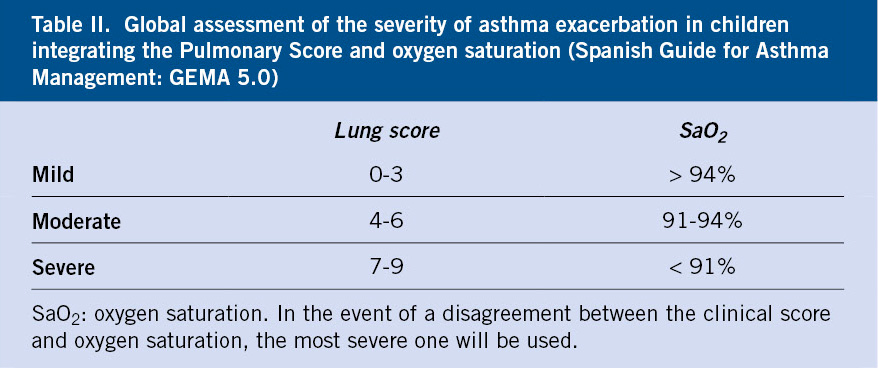
The combination of symptoms together with oxygen saturation (SaO2) will allow to estimate the severity of the episode(7) (Table II).
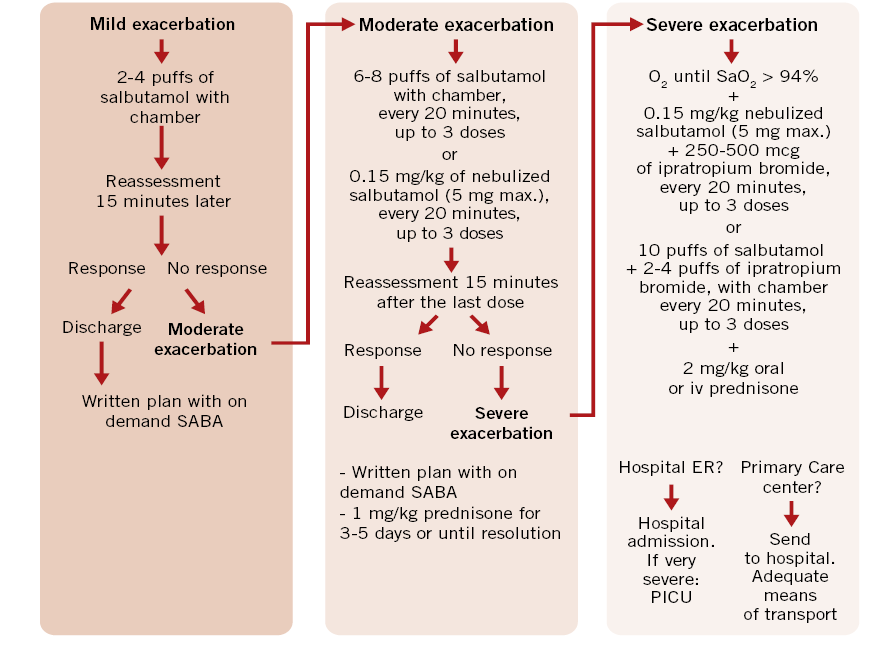
Figure 1 represents the treatment proposed by the Spanish pediatric consensus and the GEMA5.0 Guide to treat asthma attacks according to their severity(7,12).
Figure 1. Treatment of asthma exacerbation in children (Spanish Guide for Asthma Management:
GEMA5.0). kg: kilogram; min: minute; mg: milligram; µg: microgram; SaO2: oxyhemoglobin saturation; max.: maximum. SABA: Short-acting inhaled β2 adrenergic agonists.
It is advisable to individualize the dose of drugs according to the severity of the exacerbation and the response to treatment. Generally, for mild and moderate exacerbations, MDI (Metered Dose Inhaler) with chamber is preferred over nebulizer. Therefore, to avoid logistical problems, health care providers at primary care and at hospitals should encourage children and their families to bring their spacer and inhaler when attending the emergency room.
During the SARS-CoV-2 pandemic, avoiding nebulizers and using MDI with spacer, becomes even more relevant.
Medications
The main characteristics of the drugs used in the treatment of acute asthma attacks are described below.
Short-acting inhaled β2 adrenergic agonists (SABA)
They are the bronchodilator drugs with the greatest effectiveness, onset of action and fewer side effects, which is why they constitute the first line of treatment(13).
In children, salbutamol is mainly used in primary care centers and hospitals, however the patient can use terbutaline if prescribed, unless the asthma attack is so severe that it prevents him/her from properly inhaling through the turbuhaler system, which is how the latter drug is marketed in Spain.
Ipratropium bromide
Together with rescue β2 agonists, it is indicated during the first 48 hours of a severe asthma attack. Its early use has been associated with a decrease in the number of hospitalizations. Its onset of action is slower, between 30 and 60 minutes. The nebulized dose is 250 µg / 4-6 hours in patients under 30 kg and 500 µg / 4-6 hours in patients over 30 kg(15). The inhaler (with chamber) dose is 40-80 µg / 4-6 hours (2-4 puffs).
Systemic glucocorticoids
They are used for the treatment of moderate or severe exacerbations in short schemes (3-5 days or until resolution). In this case, they are administered at a dose of 1-2 mg / kg / day of prednisone or equivalent, with a maximum of 50 mg / day, as a single morning dose. Oral route is preferred, whenever possible(8). Steroids used during short periods can be withdrawn abruptly, as they seem not to affect the hypothalamus-pituitary-adrenal axis.
Alternatively, dexamethasone can be used. The effect of administering a single dose of dexamethasone orally (0.3 mg / kg) is not inferior to that of prednisolone at a dose of 1-2 mg / kg / day (maximum 40 mg) for 3 to 5 days, or up to resolution(16).
With regards to the additional use of inhaled glucocorticoids, there is currently insufficient evidence to recommend it(17).
Evaluation and maintenance treatment
One of the novelties of GEMA5.0 when initiating maintenance treatment relates to the asthmatic terms: occasional episodic, frequent episodic, moderate persistent or severe persistent, when classifying asthma the first time in a child who does not receive preventive treatment. These terms are replaced by mild, moderate or severe asthma, depending on the clinical assessment of the patient(7).
In case the child has mild and infrequent daytime symptoms, does not have inter-exacerbation symptoms, tolerates exercise well and does not present nocturnal symptoms, he or she will be allowed to receive only short-acting β2 adrenergic bronchodilators on demand. In all other cases, an anti-inflammatory maintenance treatment will be commenced.
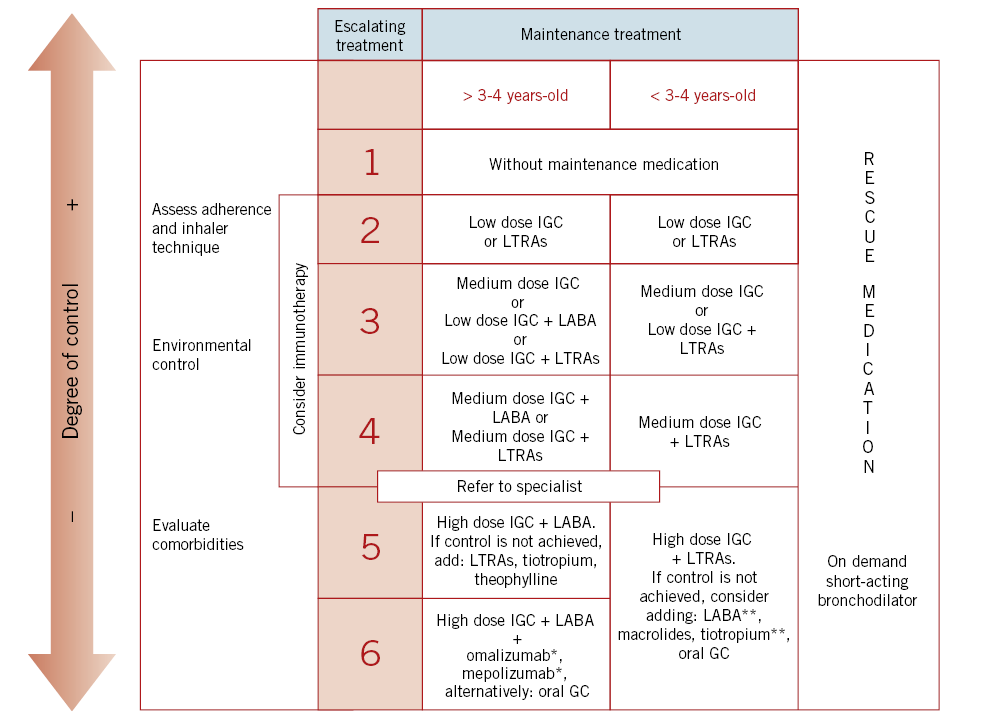
Figure 2 shows escalation of treatment in the pediatric age depending on its degree of control (GEMA5.0).
Figure 2. Escalation treatment of asthma in pediatric age depending on the degree of control (Spanish Guide for Asthma Management: GEMA5.0 ). IGC: inhaled glucocorticoids; LTRAs: Leukotriene receptor antagonists; LABA: long-acting β2 adrenergic agents; GC: glucocorticoid; *: from 6 years-old; **: off indication.
To facilitate the evaluation of symptom control, 2-3 months after commencing treatment, specific questionnaires can be offered to the parents and patient, the purpose of which is to try to verify the response to this initial treatment.
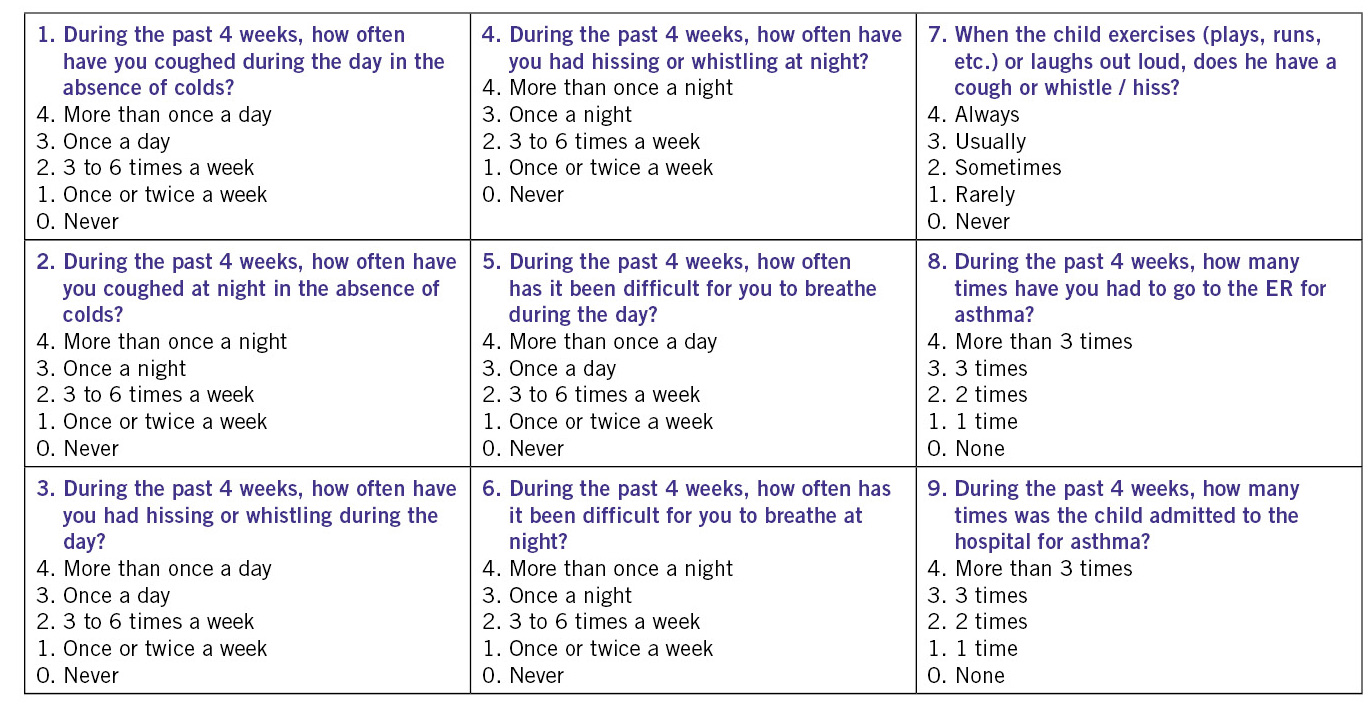
The Child Asthma Control (CAN) questionnaire (Fig. 3a), considers a patient to be poorly controlled when he or she has a score equal to or greater than 8 points(7).
Figure 3a. Asthma Control Questionnaire in Children (CAN).
Another available questionnaire is the c-ACT (Childhood Asthma Control Test) validated in Spanish (Fig. 3b).
Figura 3b. Pediatric Asthma Control Test (ACT) [also available validated in Spanish (Spanish Guide for Asthma Management: GEMA5.0)].
This questionnaire is aimed at children between 4-11 years-old and consists of 7 questions, 4 aimed at children (answers expressed with smiley icons) and 3 at parents / caregivers. A patient is considered to be poorly controlled when the score is below 20(7). The ACT for over 12-year-olds consists of 5 questions, which are completed only by the adolescent and it considers poorly controlled asthma when the score is 20 or less, and well but insufficient control from 21 to 24 points.
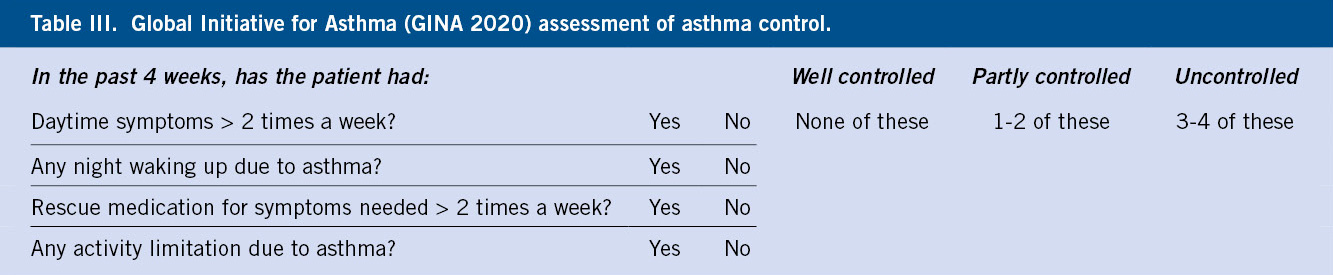
GINA recommends a shorter questionnaire of only four questions for asthma control(8) (Table III).
If after frequent clinical reviews to ensure adherence to treatment, correct use of inhalers, and if after two to three months asthma is not controlled, escalation of treatment must be considered. Of course, the correct diagnosis must have been confirmed and modifiable risk factors addressed.
After three months of total asthma control, reduction of treatment can be contemplated. The decrease will be gradual, for instance, in the opposite direction to how it was ascended, although it shall be individualized based on the response obtained to different drugs. For example, for those patients who were not responders to leukotriene receptor antagonists in monotherapy, it would not make sense now to leave them on it again.
Inhaled glucocorticoids (IGC)
They are the recommended first line treatment from stage 2 of asthma in the pediatric age(7).
Given their high affinity and selectivity for the receptor, they allow a powerful local anti-inflammatory effect, sustained therapeutic actions, prolonged presence in the lung and low oral bioavailability. They reduce asthma symptoms and the number of exacerbations.
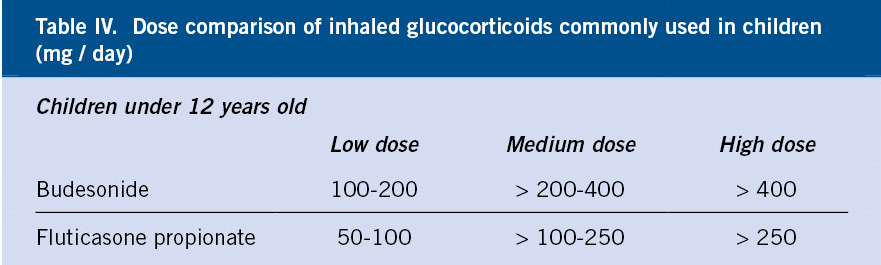
The available IGC in Spain are: beclomethasone dipropionate, budesonide, fluticasone propionate, fluticasone furoate, mometasone furoate and ciclesonide (the latter 3 are only authorized in Spain for age 12 years and above). Budesonide and fluticasone are the most widely used in current consensuses. The lowest effective dose of IGC should always be used(18,19).
Table IV shows the comparable doses of fluticasone propionate and budesonide(7).
Leukotriene receptor antagonists (LTRAs)
The only drug of this group authorized in Spain for children (from six months of age) is montelukast. It is used orally and in a single nightly dose. Its metabolism does not seem to be altered by large or fatty meals. LTRAs are considered to improve exercise-induced and allergen-induced asthma.
In preschoolers with asthma or viral-triggered episodes, they modestly reduce symptoms and the need for oral glucocorticoids.
In addition to inhaled corticosteroids, they appear to improve lung function and reduce the number of exacerbations. When evaluating the usefulness of antileukotrienes combined with inhaled corticosteroids, a complementary anti-inflammatory effect has been observed, which allows the reduction of the corticosteroid dose. This effect seems inferior to that observed in the association of long-acting β2 adrenergic bronchodilators with corticosteroids. If symptoms are not controlled with low-dose IGC, increasing the dosage of IGC to medium dose is more effective than its combination with montelukast. In monotherapy, it also seems to have a beneficial effect, but inferior to inhaled corticosteroids(20).
Association of long-acting β2 adrenergic agonists (LABA) and inhaled glucocorticoids (GCI)
They must always be used in combination with an inhaled glucocorticoid and must never be administered as monotherapy.
Its use in Spain is authorized for children over the age of 4 years. The following combinations are available(19): salmeterol / fluticasone propionate (from 4 years-old onward), formoterol / budesonide (from 6 years-old onward), formoterol / fluticasone propionate (from age 12 years), vilanterol / furoate fluticasone (from age 12 years) and formoterol / beclomethasone (from 18 years of age).
The recommended formoterol dose is 4.5 – 9 μg, twice a day; whereas it is 50 μg, twice daily, for salmeterol. Currently, they are not recommended as rescue medication for childhood asthma.
Long-acting muscarinic receptor antagonists (LAMA)
In this group, tiotropium bromide stands out. It has beneficial effects in the maintenance treatment of asthma, through selective and prolonged blockade of M3 receptors.
It can be used in children from 6 years of age with severe asthma that is poorly controlled with high dose IGC plus a LABA(19). The dose is 5 µg per day.
Theophylline
In monotherapy, it is less effective than IGC, although its anti-inflammatory effect allows the possibility of individually using it in cases of severe asthma(7).
Monoclonal antibodies
Anti-IgE (Omalizumab)
It has shown therapeutic efficacy (reduction of IGC dose, improvement of quality of life, reduction of exacerbations and hospitalizations) in IgE-mediated asthma in children from 6 years of age with severe asthma (step 6), insufficiently controlled with high doses of GCI and LABA(21,22). It is subcutaneously administered every 2-4 weeks with dose-titration according to total IgE and body weight.
Anti-IL5 (mepolizumab)
Pediatric population studies are currently very scarce. Despite this, Mepolizumab (anti-IL5) is recommended in children from 6 years of age with severe eosinophilic asthma, insufficiently controlled with high doses of IGC and LABA(22).
Immunotherapy
Immunotherapy reduces symptoms, rescue and maintenance medications, and bronchial hyperresponsiveness (both specific and nonspecific), provided that biologically standardized extracts are used and in appropriately selected sensitized patients(7).
In our country, approximately 10% of children are asthmatic(2) and 85% of them have an allergic etiology(7,19). Asthma triggered by an allergic mechanism will be treated in the same way as asthma triggered by other causes, but in addition, avoidance of specific allergens and the therapeutic option of immunotherapy should be taken into account,.
Subcutaneous or sublingual immunotherapy, with allergen vaccines, is an effective treatment for well-controlled allergic asthma (steps 2-4), provided that clinically relevant IgE-mediated sensitization to common aeroallergens has been demonstrated. Immunotherapy should not be prescribed to patients with uncontrolled asthma, due to the high risk of serious adverse reactions. Subcutaneous immunotherapy should only be administered by trained personnel and in centers where the necessary means are available to treat potential anaphylaxis. The patient should be observed for 30 minutes after the subcutaneous injection; since it is then when the rare, although possible, serious reactions have been described.
Immunotherapy prevents the development of new sensitizations and asthma in children with rhinitis(23).
Treatment of exercise-induced bronchospasm
It is essential to convey to the child and his parents the fact that, with general measures and treatment, he will be able and should continue to exercise.
When a child presents with exercise-induced bronchospasm, the most frequent possibility is that it is a not completely controlled asthma, and once other diagnoses have been ruled out, the baseline treatment should be increased.
When the child or adolescent exclusively presents symptoms with physical exercise, a preventive treatment must be indicated.
Sedentary lifestyle should be avoided and the adolescent should be explained how a proper treatment will prevent the appearance of symptoms(24). Fitness and aerobic capacity should necessarily improve. Practicing sports will be beneficial for the progression of asthma, if performed properly. Certain sports, such as swimming, are usually better tolerated and, in addition, improve lung function in adolescents and children. Other recommendations include: an adequate and progressive warm-up beforehand, progression of exercise, the use of scarf if the environment is cold and dry, and avoidance of mouth breathing as much as possible.
Regarding medications, short-acting β2 are recommended, 10-15 minutes prior to exercise. When used very frequently or continuously, a progressive loss of effectiveness may develop (tachyphylaxis), thus in these cases, the recommendation is to associate maintenance inhaled corticosteroids. Likewise, in children in whom the timing of physical exercise is unpredictable, a baseline treatment is also recommended. Antileukotrienes are beneficial in a non-negligible percentage of these patients, hence a therapeutic trial can be performed(20).
Inhalation delivery devices
Regardless of the child’s age, the inhaled route is the most suitable for administering medication in the treatment of asthma.
No pharmacological treatment with inhaled medication can be delivered correctly, if the child (whether young or adolescent) and family do not use the different inhalation systems correctly(25).
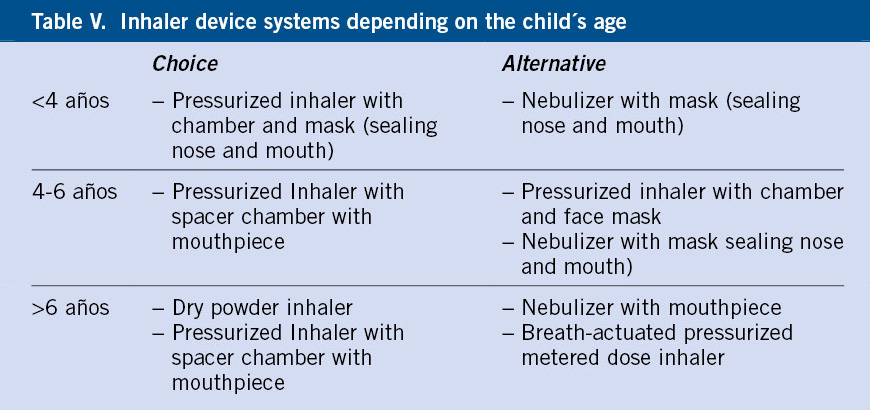
Table V serves as a guide to the inhalation devices recommended according to age. Between nebulizers or inhalers with chamber/spacer, the latter system is preferred, relegating nebulizers for very specific cases of young, uncooperative children.
Periodic review of the inhalation technique is necessary, as well as the consideration to transition from one system to another, depending on age, preferences of the older child, or whenever asthma does not evolve correctly.
Asthma education
Educating the child with asthma and his family increases the quality of life, and reduces the risk of exacerbations and healthcare costs, which is why it is one of the fundamental pillars of treatment.
The Primary Care pediatrician is key in the education of the asthmatic child
The educational approach is responsibility of all health professionals: pediatricians, pediatric allergists and pulmonologists, nurses, physiotherapists and pharmacists. However, the Primary Care pediatrician and the health center nurse, due to their proximity, accessibility and trust, play a fundamental role(12,18).
Having an accessible pediatrician, who provides continuous care to the child from birth and knows the patient’s socio-family environment(26) and his illness, will make the health center the ideal setting to respond to educational and health needs, including control of his asthma.
At present, the role of new technologies, teachers and trainers trained in asthma and the so-called “expert patient” that, through groups or associations of patients, can be useful in the education of children and their families, cannot be ignored, albeit these require supervision by health professionals(26,27).
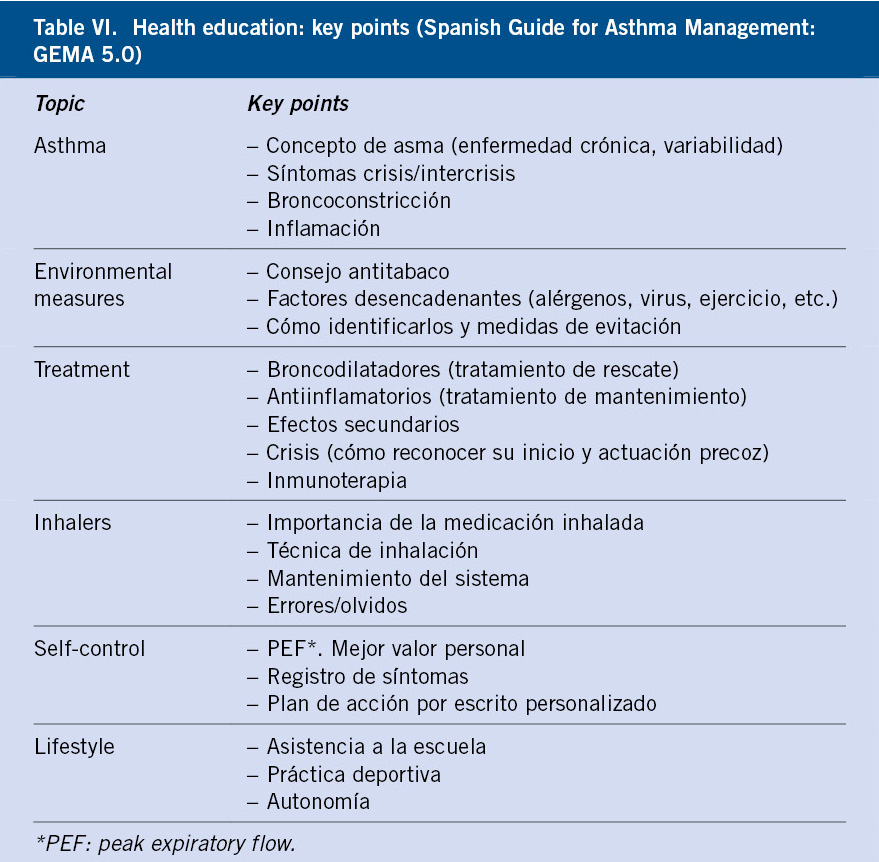
The key points to educate on are specified in table VI.
Objectives and educational sequence
For education to be effective, it is essential to identify the educational needs and the factors that impact the behavior of the patient and / or his family.
After the educational diagnosis and the identification of the needs and, depending on them and the available resources, the objectives must be established based on agreement between the child, his family and the educator(26-28). The general objective of education is to increase the quality of life of the child or adolescent and their families. Among the specific objectives are, in addition to the adequate training of the healthcare personnel involved in the program: to improve communication between patients and healthcare personnel, reduce anxiety, clarify doubts and overcome false beliefs and expectations. By increasing the knowledge of the child and his family, the aim is to induce behavioral changes and skills they need to: reduce the number of visits to the emergency department, avoid hospital admissions, improve clinical symptoms, develop prevention behaviors by identifying triggering factors, handle his condition according to his needs and future projects, and ultimately, improve quality of life in the short and long term(29).
Therefore, by means of a structured methodology termed “educational sequence”, the contents will be applied and developed in the following stages:
• Educational diagnosis.
• Awareness of illness and possible risks.
• Adherence to information. The way in which the information is presented and the empathy are essential in this stage.
• Search for solutions.
Given that asthma is a chronic disease, at this stage it is essential that the pediatrician abandons the role of expert, and switches to a more horizontal model, where solutions are sought by mutual consensus, agreeing to changes in habits and behavior modifications that promote autonomy of the child or adolescent.
In each visit, the maintenance treatment, the inhalation technique, recognition of the symptoms and approach in the event of a possible exacerbation will be reminded. It is essential that the educational program is developed in the first 6 months after diagnosis and, a minimum of three educational sessions to train and empower the child in a personalized self-management program are considered necessary(29).
Other relevant aspects include: making an escalated information plan, with a clear and understandable language adapted to each family, using personalized written information and based on graphic materials or instruments, such as chambers, placebos, explanatory rings on inflammation or bronchoconstriction, which may be helpful.
Adherence to treatment
Asthma, as other chronic diseases with long asymptomatic periods, has a high rate of therapeutic non-compliance.
The degree of adherence to treatment in pediatric asthma can be defined as the extent to which the child and / or his family truly follow the advice and use the medication that is indicated and agreed with the health care personnel, and does so correctly, using adequate techniques. At the present time, there are no effective measures for the assessment of pharmacological compliance of asthma in Pediatrics.
There are no known biochemical parameters to indicate with certainty the degree of treatment compliance and optimal disease control. An indirect attempt can be made by measuring the medication consumed and prescriptions made, using the symptom diary and interviewing the patient and his family.
Bibliography
The asterisks indicate the interest of the article considered by the authors.
1. World Health Organization. Accessed July 30, 2020. Available at: https://www.who.int/news-room/fact-sheets/detail/asthma
2. Carvajal-Urueña I, García-Marcos L, Busquets-Monge R, Morales M, García de Andoine N, Batlles-Garrido J, et al. Geographic variations in the prevalence of asthma symptoms in Spanish children and adolescents. International Study of Asthma and Allergies in Childhood (ISAAC) phase III Spain. Arch Bronchoneumol. 2005; 41: 659-66.
3. Mallol J, García.-Marcos L. Solé D, Brand P; EISL Study Group. International prevalence of recurrent wheezing during the first year of life: variability, treatment patterns and use of health resources. Thorax. 2010; 65: 1004-9.
4. Pellegrini J, Miguel G, Dios de B, Vicente E, Lorente F, García-Marcos L. Study of wheezing and its risk factors in the first year of life in the Province of Salamanca, Spain. The EISL Study. Allergol Immunopathol (Madr). 2012; 40: 164-71.
5. Hansen TE, Evjenth B, Holt J. Increasing prevalence of asthma, allergic rhinoconjunctivitis and eczema among schoolchildren: three surveys during the period 1985-2008. Acta Paediatr. 2013; 102: 47-52.
6. Blasco Bravo AJ, Pérez-Yarza EG, Lázaro y de Mercado P, Bonillo Perales A, Díaz Vázquez CA, Moreno Galdó A. Cost of asthma in Pediatrics in Spain: a cost assessment model based on prevalence. An Pediatr (Barc). 2011; 74: 145-53.
7.*** GEMA5.0. Spanish guide for the management of asthma. Ed. Luzán 5, SA Madrid 2020. Accessed July 30, 2020. Available at: www.gemasma.com.
8.*** Global Initiative for asthma (GINA). Accessed July 30, 2020.Available at: http://www.ginasthma.org/.
9. Bekhof J, Reimink R, Brand PL. Systematic review: insufficient validation of clinical scores for the assessment of acute dyspnoea in wheezing children. Paediatr Respir Rev. 2014; 15: 98-112.
10. Eggink H, Brand P, Reimink R, Bekhof J. Clinical Scores for Dyspnoea Severity in Children: A Prospective Validation Study. PLoS One. 2016; 11: e0157724.
11. Smith SR, Baty JD, Hodge D. Validation of the pulmonary score. An asthma severity score for children. Acad EmergMed. 2002; 9: 99-104.
12.*** Castillo Laita JA, De Benito Fernández J, Escribano Montaner A, Fernández Benítez M, García de la Rubia S, Garde Garde J, et al. Consensus on the treatment of asthma in Pediatrics. An Pediatr (Barc). 2007; 67: 253-73.
13. Robertson CF, Smith F, Beck R, Levison H. Response to frequent low doses of nebulized salbutamol in acute asthma. J Pediatr. 1985; 106: 672-4.
14. Griffits B, Ducharme FM. Combined inhaled anticholinergics and short-acting beta2-agonists for initial treatment of acute asthma in children. Cochrane Database Syst Rev. 2013; 8: CD000060.
15. Vézina K, Chauhan BF, Ducharme FM. Inhaled anticholinergics and short-acting beta2-agonists versus short-acting beta2-agonists alone for children with acute asthma in hospital. Cochrane Database Syst Rev. 2014; 7: CD010283.
16. Mathew JL. Oral Dexamethasone versus Oral Prednisolone in Acute Asthma: A New Randomized Controlled Trial and Updated Meta-analysis: Evidence-based Medicine Viewpoint. Indian Pediatr. 2018; 55: 155-9.
17. Kearns N, Maijersl, Harper J, Beasley R, Weatherall M. Inhaled corticosteroids in acute asthma: systemic review and meta-analysis. J. Allergy Clin Immunol Pract. 2020; 8: 605-17.
18.** De Arriba Méndez S, Pellegrini Belinchón J, Ortega Casanueva. Treatment of the asthmatic child. Comprehensive Pediatric. 2016; XX (2): 94-102.
19.** Torres Borrego J, Ortega Casanueva C, Tortajada-Girbés M. Treatment of pediatric asthma. treatment of asthma attack. Diagnostic protocol pediatr. 2019; 2: 117-32.
20. Hussein HR, Gupta A, Broughton S, Ruiz G, Brathwaite N, Bossley CJ. A meta-analysis of montelukast for recurrent wheeze in preschool children. Eur J Pediatr. 2017; 176: 963-9.
21. Corren J, Kavati A, Ortiz B, Colby JA, Ruiz K, Maiese BA, et al. Efficacy and safety of Omalizumab in children and adolescents with moderate-to-severe asthma: A systematic literature review. Allergy Asthma Proc. 2017; 38: 250-63.
22. Ahmed H, Turner S. Severe Asthma in children-a review of definitions, epidemiology and treatment options in 2019. Pediatr Pulmonol. 2019; 54: 778-87.
23. Kristiansen M, Dhami S, Netuveli G. Allergen immunotherapy for the prevention of allergy: A systematic review and meta-analysis. Pediatr Allergy Immunol. 2017; 28: 18-29.
24.*** Ortega Casanueva C, Pellegrini Belinchón J, De Arriba Méndez S. Asthma and adolescence. Adolescere. 2018; VI (3): 14-26.
25.*** Ortega Casanueva C, Pellegrini Belinchón J, de Arriba Méndez S. Inhalation devices in inhaled medication. Diagnostic protocol pediatr. 2019; 2: 51-64.
26. Pinnock H, Parke HL, Panagioti M, Daines L, Pearce G, Epiphaniou E, et al. Systematic meta-review of supported self-management for asthma: a health care perspective. BMC Med. 2017; 15:64.
27.*** GEMA educators. Asthma Educator Manual. Madrid: Luzán 5. 2010.
28. Ortega Casanueva C, Pellegrini Belinchón J. Asthma: health education, self-control and preventive measures. Comprehensive Pediatrics. 2012; XVI (2): 141-8.
29. Gillete C, Rockich-Winston N, Shepherd M, Flesher S. Children with asthma and their caregivers help improve written asthma action plans: A pilot mixed-method study. J. Asthma. 2008; 55: 609-14.
Recommended bibliography
- GEMA5.0. Spanish guide for the management of asthma. Ed. Luzán 5, SA Madrid 2020. Accessed October 15, 2020. Available at: www.gemasma.com.
Essential for the management of this pathology, both in adults and children. Consensus guide developed by 17 Spanish scientific societies and groups, and with an international scope. The pediatric part was agreed by: SEPEAP, SEN, SEICAP and AEPap. Updated in May 2020, it provides the latest available evidence and expert consensus.
- Castillo Laita JA, De Benito Fernández J, Escribano Montaner A, Fernández Benítez M, García de la Rubia S, Garde Garde J, et al. Consensus on the treatment of asthma in Pediatrics. An Pediatr (Barc). 2007; 67: 253-73.
Childhood asthma consensus developed by 5 Spanish Scientific Societies related to asthma: SEN, SEICAP. SEPEAP, AEPap and SEUP. It describes in detail with the education of children and their families, as a fundamental basis for the treatment of asthma.
- GEMA educators. Asthma Educator Manual. Madrid: Luzán 5. 2010.
It is an essential guide to approach asthma education.
- Global Initiative for asthma (GINA). Accessed October 25, 2020. Available at: http://www.ginasthma.org/.
International consensus on the diagnosis and treatment of asthma, prepared by the National Heart, Lung and Blood Institute of the USA, with the collaboration of specialists representing different parts of the world.
- Ortega Casanueva C, Pellegrini Belinchón J, De Arriba Méndez S. Asthma and adolescence. Adolescere 2018; VI (3): 14-26.
The treatment of asthma in adolescents is addressed in a detailed and updated manner.
- Ortega Casanueva C, Pellegrini Belinchón J, de Arriba Méndez S. Inhalation devices in inhaled medication. Diagnostic protocol pediatr. 2019; 2: 51-64.
Updated review of the inhalation devices approved and marketed in Spain, along with the systematics for their handling.
| Clinical case |
|
21-month-old infant with episodes of coughing and wheezing coinciding with catarrhal processes, predominantly in autumn and winter. Family history: healthy mother with no relevant history. Father has atopic dermatitis, rhinoconjunctivitis and allergic asthma due to Alternaria fungus, currently receiving immunotherapy treatment. Three-year-old brother has cow’s milk allergy and is under desensitizing therapy. Personal history: adequately vaccinated, negative neonatal metabolic screening results (including cystic fibrosis). RSV positive bronchiolitis at 3 months of age, requiring PICU admission and non-invasive mechanical ventilation for 72 hours. Afterwards, during the first year of life he presented episodes of wheezing during four catarrhal episodes, manifesting good response to salbutamol. Ever since, he has received fluticasone (50 micrograms every 12 hours in a spacer chamber). In the following fall, she attended her pediatrician again because she was wheezing three times a month approximately. Likewise, his mother stated that at the beginning of September, the boy had an asthma attack, whose symptoms lasted 11 days, and that required ER care. He was then treated with salbutamol and methylprednisolone. After confirming correct adherence and inhaler technique, we increased the dose of fluticasone to 100 micrograms every 12 hours. In the following reviews up to now, three months later, we verify that he continues to be fully controlled.
|
 New approach in the treatment of children with asthma
New approach in the treatment of children with asthma