|
| Topics on Continuous Training |
V. Losa Frías, M. Herrera López, I. Cabello García, P.I. Navas Alonso
*Fuensalida Primary Care Center. Toledo. **Pediatric Service. Virgen de la Salud Hospital. Toledo. ***La Puebla de Montalbán Primary Care Center. Toledo. ****Pedro Fuente Primary Care Center. Bargas. Toledo
| Abstract
Cancer in childhood has a low incidence and its clinical presentation is often nonspecific simulating common pathologies of benign course, therefore making its diagnosis highly challenging. The main symptoms and signs of suspected childhood cancer in Primary Care are: pallor, masses (in the head, neck and other locations), lymphadenopathy, abnormal movement, bruising and signs of bleeding, fatigue, headache, visual abnormalities, pain and musculoskeletal symptoms. The Primary Care pediatrician must recognize when an apparently benign symptomatology may be the beginning of a severe pathology, identifying those warning signs that require immediate assessment. A detailed medical history, a complete physical examination and a clinical follow-up are essential. The objective is to reduce the time from the onset of symptoms to the final diagnosis, so as to allow an early diagnosis of childhood cancer. |
Key words: Lactante; Niño; Neoplasias; Atención Primaria; Diagnóstico precoz de cáncer.
Palabras clave: Infant; Child; Neoplasms; Primary health care; Early detection of cancer.
Pediatr Integral 2021; XXV (6): 283 – 295
How to suspect cancer in
Primary Care
Introduction
Childhood cancer has a low incidence and often manifests nonspecifically, simulating other frequent and benign processes. These particularities make it difficult to diagnose cancer in Primary Care. However, an early suspicion, together with a rapid referral of the patient to a specialized center, may have implications at the prognostic and therapeutic level, as well as in the emotional impact secondary to the diagnosis on the patient and his family.
Epidemiology
Childhood cancer is after accidents, the second leading cause of death beyond the first year of life.
The standardized annual incidence of childhood cancer in Spain is 159 new cases per year per million children aged 0 to 14 years, which represents 1,100 new cases of childhood cancer per year, an incidence similar to that of the rest of Europe(1). It is estimated that a Primary Care pediatrician following 1,500 patients in his clinic, will see a new case of cancer every 5 years. Overall 5-year survival after the diagnosis is around 79%(1). Despite recent advances, childhood cancer is the second leading cause of death from the first year of life through adolescence. In the year 2018, 192 children under 14 years of age died in Spain from cancer, which means 4 children died per week due to this cause(2). The most frequent neoplasms from birth to 14 years of age are: leukemias (28%), central nervous system (CNS) tumors (23%) and lymphomas (12%), with a distribution pattern by sex and age similar to that of the rest of Europe, whilst between 15 and 19 years of age they are: bone tumors (24%), lymphomas (21%) and CNS tumors (15%). The most frequent diagnoses according to the age group are shown in Table I(1).
Patients at risk
Medical history is the most effective tool in identifying cancer predisposition syndromes.
There is a hereditary basis in 8-10% of all neoplasms. Within this percentage, cancer predisposition syndromes (CPS) are included, conforming a heterogeneous group of genetic conditions and immunodeficiencies, which predispose to a greater risk of cancer (Table II).
Most of these syndromes are rare and show variable expressiveness within the family members. It is important to identify these patients, as they can benefit from prevention and early detection measures, as well as the possibility of genetic counseling. During history-taking, CPS may be suspected should there be(3):
• Several cases of cancer within the family, usually of the same type.
• Multi-generational disease cases, presenting at an earlier age than in the general population.
• Presence of tumors in association with developmental defects: generalized or asymmetric body overgrowth, dysmorphic features, congenital malformations or mental retardation.
• Presence of bilateral or multifocal tumors.
• Individuals with more than one primary tumor.
• Presence of rare, benign tumors or cysts associated with CPS.
Warning signs and symptoms
Apparently benign symptomatology, but with atypical presentation or torpid course, may be the beginning of a neoplastic process.
Childhood cancer can manifest in its initial stages, with symptoms similar to frequent and benign processes(4). The purpose is to recognize when this apparently benign symptomatology may be the beginning of a serious pathology, as well as to identify those findings (red flags) that, in combination with the rest of the data from history and physical examination, should alert us of the possibility of cancer (Table III).
For this, it is necessary to listen and pay special attention to parents(5), who in general are the best observers of their children’s symptoms, and also to adolescents(6); taking a complete medical history including personal and family history, and a thorough physical examination.
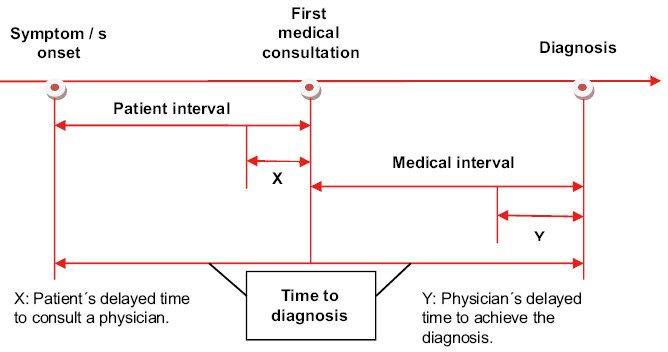
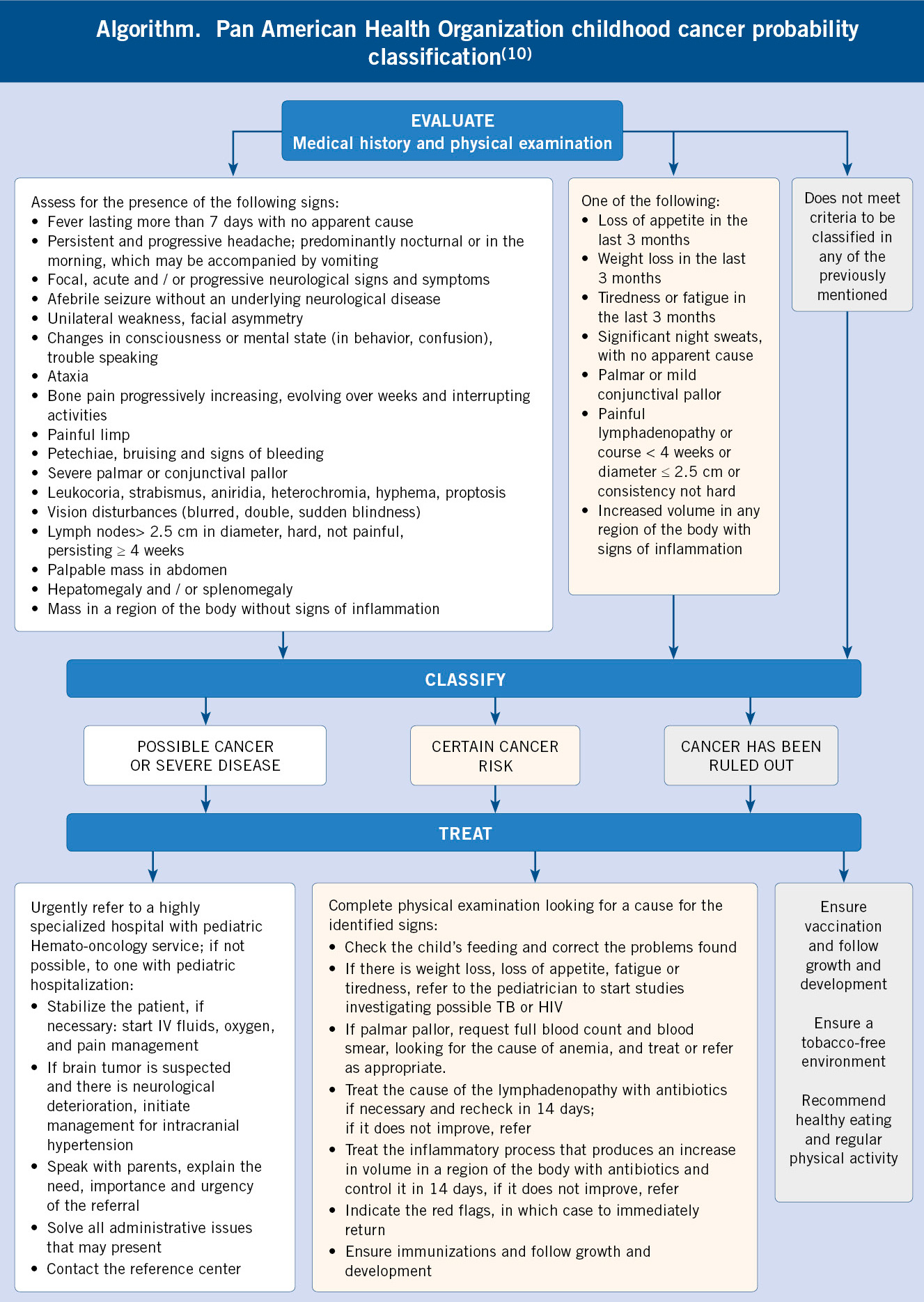
Qualitative studies highlight the importance of behavioral and affective changes detected by parents, that motivate the first visits to Primary Care prior to the diagnosis of cancer, sometimes in the absence of other signs of alarm(5,7). Such is the case, that the National Institute for Clinical Excellence (NICE) recommends considering the persistent parental concern regarding their children’s symptoms as a reason for study or referral(8). In Primary Care, the following signs and symptoms have been reported to increase the possibility of cancer: paleness, head and neck masses, abdominal masses, lymphadenopathy, motor abnormalities, bruises and other signs of bleeding, asthenia, headache, visual anomalies, pain and musculoskeletal symptoms. However, except for abdominal masses, the positive predictive value of these symptoms is low, given the low frequency of childhood cancer. Even so, given the severity of the diagnosis, the presence of the aforementioned symptoms, fundamentally when it occurs without a clear cause and leads to an increase in the number of consultations (3 or more in a period of 3 months), should alert of the possibility of a neoplastic process(9). In this sense, the Pan American Health Organization has published an evaluation strategy, a cancer probability classification and attitude based on the findings of the history and examination shown in Algorithm 1(10).
Headache and other neurological signs and symptoms
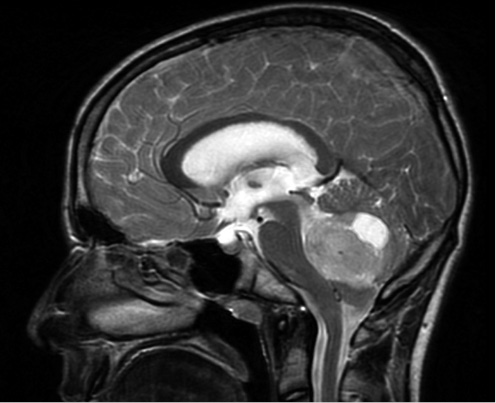
Primary CNS tumors are the second most common neoplasia in childhood after leukemias(1), as well as the second leading cause of death from childhood cancer(2). Their symptoms are due to the invasion and compression of the adjacent nervous tissue, as well as the increase in intracranial pressure due to mass effect or obstructive hydrocephalus (Fig. 1), manifesting a very heterogeneous clinical presentation.
Figure 1. Sagittal MRI showing a mass in the posterior fossa compatible with medulloblastoma. The 13-year-old patient presented with headache and papilledema.
Wilne et al.(11) analyzed 74 articles (n = 4,171), identifying up to a total of 56 signs and symptoms at the diagnosis of a CNS tumor, which depended on age, location and history of neurofibromatosis (NF). For intracranial tumors, excluding NF, the most frequent symptoms were: headache, nausea and vomiting, abnormality of gait and coordination, and papilledema. In intracranial tumors associated with NF these were: decreased visual acuity, exophthalmos, optic atrophy and strabismus. In intracranial tumors in children under 4 years they were: macrocephaly, nausea and vomiting, irritability, lethargy and ataxia. And in spinal cord tumors they were: back pain, gait and coordination abnormality, spinal deformity, focal weakness and sphincter alterations. Given this clinical variability, they subsequently studied the evolution of symptoms in a retrospective cohort (n = 139), describing a progressive increase in the number of symptoms. Thus, half of the patients went from one symptom at the beginning of the process to six at the diagnosis of the tumor(12). In Primary Care, we must be alert to patients with non-resolving symptoms or in which new ones are associated, especially: visual, motor, endocrine or behavioral, as well as signs of intracranial hypertension(12-14). In this line, Ansell et al.(15) described the reasons for consultation in Primary Care, from birth to the diagnosis of a CNS tumor in a series of patients, comparing it with a control group. They observed how the cases consulted three times more often for a sign or symptom suggestive of a CNS tumor, reaching seven times more when they associated two or more symptoms.
The Children’s Brain Tumour Research Centre group has developed an evidence-based clinical guide(16) (Table IV), as well as the awareness strategy “HeadSmart: be brain tumours aware” (https://www.headsmart.org.uk/), which has shown positive results in reducing the time from the onset of symptoms to diagnosis (median 14 to 6.7 weeks), as well as the time from the first consultation to an imaging test (3.3 to 1.4 weeks)(17).
Fever and constitutional symptoms
Fever is one of the most frequent reasons for consultation in Pediatrics, being, in most cases, of infectious etiology. Only 6% of the cases of fever of unknown origin correspond to neoplasms(18). This fever can be of tumor origin (Ewing’s sarcoma, neuroblastoma, Hodking’s lymphoma…) or due to infections secondary to the alteration of the immune system due to cancer, as can occur in leukemias.
Leukemia is the most common pediatric tumor(1). Clarke et al.(19) analyzed the symptoms at the diagnosis of leukemia in childhood and adolescence in 33 studies (n = 3,084), and identified a total of 95 signs and symptoms, of which five were present in more than half of the patients: hepatomegaly, splenomegaly, paleness, fever and bruising. In addition, between a third and a half of the patients presented: recurrent infections, asthenia, pain in the extremities, hepatosplenomegaly, hematomas / petechiae, lymphadenopathies, bleeding tendency and skin rash. These findings highlight the importance of performing a complete physical examination in children with common symptoms, such as fever, but with a torpid or persistent course, paying special attention to abdominal palpation, lymphadenopathy search and careful skin examination. Despite the fact that leukemia is the most frequent pediatric cancer, there is currently no evidence of the predictive value of clinical data at the individual level, or of their combination. NICE guidelines recommend that pediatric patients with fever of unclear cause or in combination with unjustified clinical data such as: paleness, asthenia, lymphadenopathies, splenomegaly, osteoarticular pain, bruising, night sweats or weight loss, should be evaluated with a full blood count and peripheral blood smear within 48 hours; in the case of associating petechiae or unexplained hepatosplenomegaly, immediate referral is recommended(8).
Lymphadenopathies
Lymph nodes are dynamic structures that change in size during children´s growth, usually in response to infections. During childhood, palpation of small lymph nodes in cervical, axillary, or inguinal regions is normal. An increase in size above 1 cm in cervical and axillary nodes, 1.5 cm in inguinal and 0.5 cm in epitrochlear nodes, as well as stone-like consistency, irregular surface, existence of skin ulceration or fixation is considered pathological(20) (Fig. 2).
Figure 2. Mediastinal widening in a 12-year-old patient diagnosed with Hodgkin’s lymphoma. Clinically, she had multiple adherent, non-tender and larger than 2 cm laterocervical and supraclavicular lymphadenopathies.
Lymphadenopathies are generalized, when they extend beyond more than 2 non-contiguous ganglion chains, and localized, when they appear in a single region. According to the course in terms of length of time, we distinguish between acute (less than three weeks) and subacute / chronic (more than three weeks / months)(21). In the history, we should inquire for: the age of the patient, the form of onset, the time of progression and the speed of growth, as well as the presence of recent or recurrent infections, contact with sick people, associated symptoms, previous antibiotic treatments, similar episodes, vaccination status, medications, contact with animals or recent travels. A complete physical examination should be performed, looking for signs of systemic disease and paying special attention to the presence of skin lesions, paleness, signs of bleeding, oropharyngeal or conjunctival lesions, hepatosplenomegaly, and abdominal masses. Adenopathies will be evaluated based on their size, location, tenderness, consistency, mobility, local inflammatory signs, and presence of skin fistulas. We must systematically palpate all accessible ganglion chains: occipital, retroauricular, preauricular, parotid, tonsillar, submandibular, submental, anterior and posterior neck, supraclavicular, infraclavicular, axillary, epitrochlear, inguinal and popliteal. Warning signs in the evaluation of adenopathies are: size greater than 3 cm, rapid growth in the absence of inflammatory signs, hard consistency, fixation to deep planes, supraclavicular, axillary, generalized or confluent location, as well as constitutional symptoms, the presence of abdominal masses, hard hepatosplenomegaly, signs of respiratory distress, paleness, jaundice or bleeding(8,20-22).
Mediastinal mass
Mediastinal masses in childhood are rare and, in most cases, are malignant. The most frequent location is the anterior mediastinum and the most frequent etiology is lymphoma(23,24). A thorough clinical evaluation and a high index of suspicion are important for an early diagnosis. Upon clinical suspicion, immediate referral to a hospital center to complete the study is recommended(8,23).
From an anatomical point of view, the mediastinum is divided into three compartments: anterior, middle, and posterior. The location of the mass will guide the diagnosis (Table V).
The most frequent neoplasms according to their location are: acute lymphoblastic leukemia and T lymphoma (Fig. 3) in the anterior mediastinum; Hodgkin lymphoma in the middle mediastinum; and neurogenic tumors (neuroblastoma and ganglioneuroma) in the posterior mediastinum. Posterior mediastinal masses (neuroblastoma) are more common in infants and young children, while anterior mediastinal masses (leukemias, lymphomas) are more common in older children and adolescents(24).
Figure 3. Anterior mediastinal mass in a 12-year-old boy with T-leukemia. He reported a 3 day course of asthenia and bilateral eyelid edema, as well as 48 hours of bruising, ecchymosis and petechiae in face, neck and upper arm.
A high percentage of patients are asymptomatic at diagnosis. In symptomatic cases, the symptoms are secondary to compression of adjacent structures, so the symptoms depend on the location of the mass, its size, and the rate of growth. Compression of the airway is the most frequent symptom, giving rise to nonspecific symptoms, such as: stridor, non-productive cough, wheezing, recurrent respiratory infections, chest pain and respiratory distress that often simulate frequent respiratory diseases, such as asthma or laryngitis. Esophageal compression leads to dysphagia. Compression of the spinal cord (common in neuroblastoma) results in band or radicular back pain that increases with Valsalva, gait weakness, loss of strength and sensory and sphincter alterations. Compression of the superior vena cava (characteristic of leukemias and T lymphomas) manifests with: facial plethora, headache, blurred vision, cough, chest pain, orthopnea that increases with Valsalva, hypotension and heart failure. Compression of the phrenic leads to hemidiaphragmatic elevation. Finally, injury to the sympathetic pathway (especially neuroblastomas) can cause Horner syndrome (ptosis, miosis, and enophthalmos). And, in turn, we can find systemic symptoms secondary to the tumor process itself. Compression of the phrenic leads to hemidiaphragmatic elevation. Finally, injury of the sympathetic pathway (especially neuroblastomas) can cause Horner syndrome (ptosis, miosis, and enophthalmos). In addition, we can find systemic symptoms secondary to the tumor process itself.
We must bear in mind that treatment prior to diagnosis with systemic corticosteroids in patients with hematological malignancies may have diagnostic and prognostic implications, and precipitate serious complications, such as tumor lysis syndrome. For this reason, in patients with atypical respiratory symptoms or laryngitis in older children, performing a chest X-ray prior to starting corticosteroid treatment is recommended(23,25).
Abdominal mass
The finding of an abdominal mass is one of the most frequent forms of presentation of neoplasms in childhood. Although they may be of benign etiology, all patients with an abdominal mass must be evaluated with the suspicion of malignancy and referred to a specialized center within 48 hours(8).
An abdominal mass is often asymptomatic, and is usually detected accidentally by the parents or in a routine examination. In the history, we will take into account the patient’s age (Table VI), the associated symptoms, their intensity and duration, taking into account which rapidly evolving symptoms are suggestive of malignancy(26,27).
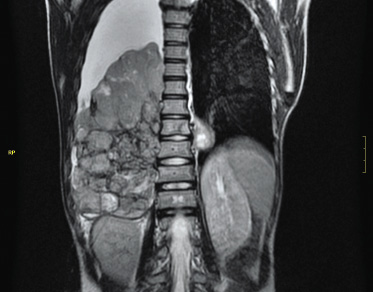
The most frequent presentation symptoms are: pain, organ dysfunction due to mass effect (intestinal or urinary obstruction), hematuria (nephroblastoma) and systemic symptoms (night sweats, fever, asthenia, weight loss or bone pain…). The most frequent etiology involves: genitourinary congenital malformations in children under one year of age; neuroblastoma (Fig. 4) and nephroblastoma (Fig. 5) between one and five years; and non-Hodgkin lymphoma (NHL) in older children and adolescents.
Figure 4. Neuroblastoma in a 7-year-old patient who reported weeks of rib pain associated with asthenia and anorexia in the most recent days.
Figura 5. Right Wilms tumor. The 2-year-old patient consulted because the parents palpated the abdominal mass while dressing her.
In this age group, Burkitt-type NHL presents as: a rapidly growing abdominal mass that associates abdominal distention and pain, obstructive symptoms, intussusception, and metabolic alterations secondary to tumor lysis(26). In girls and adolescents, we will take into account ovarian tumors and pregnancy. In the personal history, factors such as prematurity and low birth weight (hepatoblastoma), should also be assessed.
Physical examination should be performed with the patient relaxed and calm. It must be meticulous, checking vital signs, including blood pressure(27). On inspection, we will look for irregularities on the abdominal surface. On palpation, we must take into account that, in healthy patients, especially infants, some structures may be palpable, such as: liver, spleen, kidneys, abdominal aorta, sigmoid colon, feces and / or spine(28). It is important to establish the location, size, shape, and contour of the mass, its adherence to deep planes, as well as the presence of tenderness. Depending on the location it should be considered that: palpable masses in the right upper quadrant are usually of hepatic, renal or adrenal origin; those in the upper left quadrant often depend on the spleen and may be secondary to metastatic infiltration; and those located in the hypogastrium, are usually secondary to ovarian tumors or intestinal lymphomas. In the patient with suspected neuroblastoma, a complete neurological examination is necessary due to the possibility of invasion of the medullary canal. Other examination signs that can guide the diagnosis are: aniridia, hemihypertrophy and genitourinary malformations (nephroblastoma); subcutaneous nodules, periorbital ecchymoses, proptosis, intractable watery diarrhea, Horner syndrome or opsoclonus-myoclonus syndrome (neuroblastoma); precocious puberty, feminization or virilization (hepatic, gonadal, adrenal masses or germ tumors); and Cushing phenotype (neoplasms of the adrenal cortex).
Soft tissue and skin masses
Soft tissue sarcomas (STS) are a heterogeneous group of tumors that originate from primitive mesenchymal cells. They are subdivided into rhabdomyosarcoma (RBM) and non-rhabdomyosarcoma sarcomas. RBM is the most frequent, it has its origin in the primitive mesenchymal cells involved in the development of skeletal muscle, and it presents its maximum incidence around the age of five years and in adolescence. It is subdivided into: embryonal, alveolar and pleomorphic. On the other hand, non-rhabdomyosarcoma sarcomas are more common in older children and adolescents.
STS can appear in any anatomical location, the most frequent being: the genitourinary region, head and neck, and extremities. The clinical presentation depends on the location, size and adjacent structures. An unexplained tumor in any location with any of the following characteristics is suspicious of STS: diameter greater than 2 cm, fixation to deep planes, increased consistency, progressive growth, and presence of regional lymphadenopathies(4). In the head, orbital locations usually develop proptosis and a differential diagnosis must be made with benign pathologies, such as orbital cellulitis. Parameningeal locations can lead to nasal, sinus, or ear obstruction, persistent mucopurulent discharge, or cranial nerve involvement. In the genitourinary region, it can present as: hematuria, voiding syndrome, constipation, pelvic mass or increased testicular size. The botryoid variety is a subtype of embryonic RMS characterized by multiple polypoid projections that form clusters of gelatinous and friable consistency, that develop under the mucosal surface of body orifices, such as vagina and nose.
When a soft tissue tumor is suspected, NICE guidelines recommend performing an ultrasound within 48 hours(8).
Musculoskeletal symptoms and signs
Musculoskeletal pain is a frequent reason for consultation in Primary Care. Its etiology varies with age, the most frequent causes being traumatic. The differential diagnosis will include overuse syndromes and osteochondroses. Less frequently, but highly important as their diagnosis delay can increase morbidity, are neoplasms and osteoarticular infections. Among the most frequent neoplasms that present with bone and / or joint pain, primary bone tumors, neuroblastomas, NHL and leukemias, can be found.
Patients with primary bone tumors, such as osteosarcoma or Ewing’s sarcoma, frequently present with localized, persistent, asymmetric, progressive bone pain that responds poorly to common analgesics and may wake the child up at night. They can associate a palpable indurated mass fixed to deep planes and of rapid and progressive growth and, occasionally, a pathological fracture can occur. Generalized musculoskeletal pain manifests as: lower limb pain, back pain, arthralgia or arthritis. The tumors that produce it are leukemias, especially lymphoblastic leukemia and bone or medullary metastases of tumors, such as Ewing’s sarcoma or neuroblastoma.
Several authors(29-31) have highlighted the relevance of incorporating leukemias and bone tumors in the differential diagnosis of patients with suspected osteomyelitis or rheumatological diseases. Leukemias that present with joint symptoms (generally in the form of asymmetric oligoarthritis) are less frequently associated with typical leukemia symptoms, such as: constitutional syndrome, hepatosplenomegaly or cytopenia, which makes diagnosis difficult(29). In the evaluation of patients with musculoskeletal symptoms, the association of leukopenia (less than 4 x 109 / L), platelets in the lower limit of normality (150-250 x 109 / L) and a history of nocturnal pain, represents a sensitivity of 100% and specificity of 85% in the diagnosis of leukemia(30). The importance of an accurate diagnosis prior to the start of steroid treatment should be noted, given the possibility of masking a hematological neoplasm or triggering a tumor lysis syndrome. For this reason, some authors suggest performing a bone marrow study prior to initiating treatment with steroids, in those patients with suspected rheumatological disease and atypical data(31).
A complete medical history that includes: characterization of the pain (onset, location, duration, intensity, number of affected joints); presence of other accompanying symptoms (inflammation, increased temperature, joint instability); precipitating factors (previous infection or trauma); and associated systemic symptoms, as well as a thorough physical examination, will guide the diagnosis. In a patient with bone and / or joint pain with suspected cancer, an X-ray as well as a full blood count and peripheral blood smear shall be performed within 48 hours. In case of a pathological X-ray, the patient should be referred to a specialized center within 48 hours. When leukemia is suspected (cytopenia of two or more cell lines and / or blasts), referral will be immediate(8).
Eye disorders
Retinoblastoma is the most common ocular neoplasm, representing 3% of childhood tumors(1). It is usually diagnosed between the first and third year of life, and 95% of them before the age of 5 years(1). 30% are bilateral and 40% hereditary. Leukocoria is the presenting sign in more than half of the cases, and it appears as a consequence of the presence of a mass located behind the crystalline lens. In the differential diagnosis of leukocoria, in addition to retinoblastoma, congenital cataracts (history of infection in pregnancy, such as toxoplasmosis, should be investigated) and Coats disease (retinal telangiectasia with deposition of intraretinal or subretinal exudates that affects younger children). Other symptoms and signs that should alert us include: strabismus, loss of visual acuity, eye pain or proptosis. In addition to retinoblastoma, other tumors that can manifest as proptosis include: neuroblastoma, rhabdomyosarcoma, lymphoma, and histiocytosis. The successful management of retinoblastoma depends on the ability to detect the disease while it still remains intraocular(32). Hence, it is very important to test for the red reflex in all newborns and in each programmed child health visit. An abnormal result of the red reflex examination is an indication for preferential referral (in less than two weeks) to the ophthalmologist(8). Patients with a family history of retinoblastoma must be referred from birth for close ophthalmological follow-up(3).
Another ocular manifestation of cancer is the paraneoplastic opsoclonus-myoclonus syndrome, which is associated with neuroblastoma in 50% of cases. It is characterized by multidirectional, involuntary and chaotic rapid eye movements, persistent during sleep, myoclonus, ataxia, and behavioral disturbances.
Time lapse to childhood cancer diagnosis
Decreasing the time to diagnosis has prognostic implications in some childhood tumors.
The most effective strategy to reduce the morbidity and mortality of childhood cancer is to focus, both on the reduction of the time to diagnosis (TD), understood as the time elapsed from the onset of symptoms to the diagnosis of cancer(33) (Fig. 6), as in the early initiation of treatment based on scientific evidence.
Figure 6. Time to diagnosis in childhood cancer.
Modified from Lethaby et al(33).
Therefore, the recognition of alarm symptoms by families and Primary Care professionals, as well as easy access to the latter, is a priority. Screenings in childhood are usually not helpful, unless the child has a high risk of cancer associated with hereditary disorders. Among factors that have been related to TD, the following stand out: the patient’s age (the older, the greater the TD); the type of tumor (CNS, bone, germ cells and retinoblastomas have longer TD compared to leukemia and kidney tumors); and tumor biology(33-35). The relationship of TD with survival is complex. Thus, some authors(35,36) indicate that it is precisely the biology of the tumor, the factor that most influences TD and survival. In any case, there is extensive literature supporting a positive correlation between improved survival and early diagnosis in tumors such as retinoblastoma(35), being this correlation more ambiguous for CNS and other solid tumors, probably in relation to high-grade tumors having a more abrupt onset of prediagnostic symptoms and, therefore, a shorter TD(37). In the group of adolescents and young adults, although females more frequently consult physicians, they have more prolonged TD. Bone tumors and lymphomas have longer TD, probably due to the non-specific clinical presentation of these types of cancer(6).
It should be noted how, in the COVID-19 pandemic situation, several publications have warned of the increase in TD of childhood cancer in neighboring countries(38,39).
Role of the Primary Care pediatrician
The Primary Care pediatrician shares the responsibility of reducing TD, identifying those patients suspected of cancer and making an early referral to specialized care. This decrease in TD may have a prognostic role for some tumors and, in addition, contributes to the reduction of anxiety and stress experienced by patients and their families during the difficult period prior to the diagnosis of childhood cancer(5).
Bibliography
The asterisks show the interest of the article in the opinion of the authors.
1. Pardo Romaguera E, Muñoz López A, Valero Poveda S, Porta Cebolla S, Fernández-Delgado R, Barreda Reines MS PBR. Cáncer infantil en España. Estadísticas 1980-2017. Registro Español de Tumores Infantiles (RETI-SEHOP). Valencia: Universitat de Valéncia, 2018 (Preliminar edition, CD-Rom).
2. Ministerio de Sanidad, Servicios Sociales e Igualdad. Portal Estadístico (sede Web). Accessed february 28, 2021. Available at: https://pestadistico.inteligenciadegestion.mscbs.es/publicoSNS/S.
3.*** Coury SA, Schneider KA, Schienda J, Tan WH. Recognizing and managing children with a pediatric cancer predisposition syndrome: A guide for the pediatrician. Pediatr Ann. 2018; 47: e204-16.
4.*** Fragkandrea I, Nixon JA, Panagopoulou P. Signs and symptoms of childhood cancer: a guide for early recognition. Am Fam Physician. 2013; 88: 185-92.
5.*** Dixon-Woods M, Findlay M, Young B, Cox H, Heney D. Parents’ accounts of obtaining a diagnosis of childhood cancer. Lancet. 2001; 357: 670-4.
6.** Herbert A, Lyratzopoulos G, Whelan J, Taylor RM, Barber J, Gibson F, et al. Diagnostic timeliness in adolescents and young adults with cancer: a cross-sectional analysis of the BRIGHTLIGHT cohort. Lancet Child Adolesc Health. 2018; 2: 180-90.
7.*** Clarke RT, Jones CHD, Mitchell CD, Thompson MJ. Shouting from the roof tops: a qualitative study of how children with leukaemia are diagnosed in primary care. BMJ Open. 2014; 4: e004640. doi:10.1136/bmjopen-2013-004640.
8.** NICE. Suspected cancer recognition and referral: symptoms and findings. Updated january 29, 2021. Accessed april 1st, 2021. Available at: https://www.nice.org.uk/guidance/ng12.
9.** Dommett RM, Redaniel T, Stevens MCG, Martín RM, Hamilton W. Risk of childhood cancer with symptoms in primary care: a population-based case-control study. Br J Gen Pract. 2013; 63: e:22-9.
10.** Pan American Health Organization. Early diagnosis of childhood cancer. Washington, DC; OPS. 2014.
11.** Wilne S, Collier J, Kennedy C, Koller K, Grundy R, Walker D. Presentation of childhood CNS tumours: a systematic review and meta-analysis. Lancet Oncol. 2007; 8: 685-95.
12. Wilne S, Collier J, Kennedy C, Jenkins A, Grout J, MacKie S, et al. Progression from first symptom to diagnosis in childhood brain tumours. Eur J Pediatr. 2012; 171: 87-93.
13. Chu TPC, Shah A, Walker D, Coleman MP. Where are the opportunities for an earlier diagnosis of primary intracranial tumours in children and young adults? Eur J Paediatr Neurol. 2017; 21: 388-95.
14. Shanmugavadivel D, Liu JF, Murphy L, Wilne S, Walker D. Accelerating diagnosis for childhood brain tumours: An analysis of the HeadSmart UK population data. Arch Dis Child. 2020; 105: 355-62.
15. Ansell P, Johnston T, Simpson J, Crouch S, Roman E, Picton S. Brain tumor signs and symptoms: Analysis of primary health care records from the UKCCS. Pediatrics. 2010; 125: 112-9.
16.*** Wilne S, Koller K, Collier J, Kennedy C, Grundy R, Walker D. The diagnosis of brain tumours in children: A guideline to assist healthcare professionals in the assessment of children who may have a brain tumour. Arch Dis Child. 2010; 95: 534-9.
17.*** Walker D, Wilne S, Grundy R, Kennedy C, Neil, Dickson A, et al. A new clinical guideline from the Royal College of Paediatrics and Child Health with a national awareness campaign accelerates brain tumor diagnosis in UK children – “headSmart: Be Brain Tumour Aware.” Neuro Oncol. 2016; 18: 445-54.
18. Chow A, Robinson JL. Fever of unknown origin in children: a systematic review. World J Pediatr. 2011; 7: 5-10.
19. Clarke RT, Van Den Bruel A, Bankhead C, Mitchell CD, Phillips B, Thompson MJ. Clinical presentation of childhood leukaemia: A systematic review and meta-analysis. Arch Dis Child. 2016; 101: 894-901.
20.** Del Rosal Rabes T, Baquero Artigao F. Adenitis cervical. Pediatr Integral. 2018; XXII(7): 307-15.
21. Nield LS, Kamat D. Lymphadenopathy in Children: When and How to Evaluate. Clin Pediatr (Phila). 2004; 43: 25-33.
22. Chiappini E, Camaioni A, Benazzo M, Biondi A, Bottero S, De Masi S, et al. Development of an algorithm for the management of cervical lymphadenopathy in children: Consensus of the Italian Society of Preventive and Social Pediatrics, jointly with the Italian Society of Pediatric Infectious Diseases and the Italian Society of Pedi. Expert Rev Anti Infect Ther. 2015; 13: 1557-67.
23.** Green K, Behjati S, Cheng D. Fifteen-minute consultation: Obvious and not-so-obvious mediastinal masses. Arch Dis Child Educ Pract Ed. 2019; 104: 298-303.
24. Chen C, Wu K, Chao Y, Weng D, Chang J-S, Lin C-H. Clinical manifestation of pediatric mediastinal tumors, a single center experience. Medicine. 2019; 98: 32.
25.** Saraswatula A, McShane D, Tideswell D, Burke GAA, Williams DM, Nicholson JC, et al. Mediastinal masses masquerading as common respiratory conditions of childhood: A case series. Eur J Pediatr. 2009; 168: 1395-9.
26.** Uzunova L, Bailie H, Murray MJ. Fifteen-minute consultation: A general paediatrician’s guide to oncological abdominal masses. Arch Dis Child Educ Pract Ed. 2019; 104: 129-34.
27. Potisek NM, Antoon JW. Abdominal masses. Pediatr Rev. 2017; 38: 101-3.
28. Steuber PC. Clinical assessment of the child with suspected cancer. UpToDate; 2021. p. 1-24.
29. Brix N, Rosthøj S, Herlin T, Hasle H. Arthritis as presenting manifestation of acute lymphoblastic leukaemia in children. Arch Dis Child. 2015; 100: 821-25.
30. Jones OY, Spencer CH, Bowyer SL, Dent PB, Beth S, Rabinovich CE. A Multicenter Case-Control Study on Predictive Factors Distinguishing Childhood Leukemia From Juvenile Rheumatoid Arthritis. 2006; 117: e840-4.
31. Sen ES, Moppett JP, Ramanan AV. Are you missing leukaemia? Arch Dis Child. 2015; 100: 811-2.
32. Parrilla Vallejo M, Perea Pérez R, Relimpio López I, Montero de Espinosa I, Rodríguez de la Rúa E, Terrón León JA, et al. Retinoblastoma: The importance of early diagnosis. Arch Soc Esp Oftalmol. 2018; 93: 423-30.
33.** Lethaby CD, Picton S, Kinsey SE, Phillips R, Van Laar M, Feltbower RG. A systematic review of time to diagnosis in children and young adults with cancer. Arch Dis Child. 2013; 98: 349-55.
34. Barr RD. “Delays” in diagnosis: A misleading concept, yet providing opportunities for advancing clinical care. J Pediatr Hematol Oncol. 2014; 36: 169-72.
35.*** Brasme JF, Morfouace M, Grill J, Martinot A, Amalberti R, Bons-Letouzey C, et al. Delays in diagnosis of paediatric cancers: A systematic review and comparison with expert testimony in lawsuits. Lancet Oncol 2012; 13: e445-59.
36. Brasme JF, Chalumeau M, Oberlin O, Valteau-Couanet D, Gaspar N. Time to diagnosis of Ewing tumors in children and adolescents is not associated with metastasis or survival: A prospective multicenter study of 436 patients. J Clin Oncol. 2014; 32: 1935-40.
37.** Ferrari A, Lo Vullo S, Giardiello D, Veneroni L, Magni C, Clerici CA, et al. The Sooner the Better? How Symptom Interval Correlates With Outcome in Children and Adolescents With Solid Tumors: Regression Tree Analysis of the findings of a prospective study. Pediatr Blood Cancer. 2016; 63: 479-85.
38. Soares Martins QC, Gomes de Morais Fernandes FC, Pereira Santos VE, Guerra Azevedo I, Góes de Carvalho Nascimento, LS, Dos Santos Xavier CC, et al. Factors associated with the detection of childhood and adolescent cancer in primary health care: A prospective cross-sectional study. J Multidiscip Healthc. 2020; 13: 329-37.
39. Chiaravalli S, Ferrari A, Sironi G, Gattuso G, Bergamaschi L, Puma N, et al. A collateral effect of the COVID-19 pandemic: Delayed diagnosis in pediatric solid tumors. Pediatr Blood Cancer. 2020; 67: 2-3.
Recommended bibliography
- Fragkandrea I, Nixon JA, Panagopoulou P. Signs and Symptoms of childhood cancer: A guide for early recognition. Am Fam Physician. 2013; 88: 185-92.
Article focused on the early diagnosis of childhood cancer in Primary Care, by focusing on those warning signs that should alert us of the possibility of neoplastic processes.
- Clarke RT, Jones CHD, Mitchell CD, Thompson MJ. Shouting from the roof tops: a qualitative study of how children with leukaemia are diagnosed in primary care. BMJ Open. 2014; 4: e004640. doi: 10.1136 / bmjopen-2013-004640.
Highly recommended. Qualitative methodology article that explores the presentation of childhood leukemia and the factors that influence its suspicion from the point of view of parents and Primary Care physicians.
- Wilne S, Koller K, Collier J, Kennedy C, Grundy R, Walker D. The diagnosis of brain tumors in children: a guideline to assist healthcare professionals in the assessment of children who may have a brain tumor. Arch Dis Child. 2010; 95: 534-9.
A must-read. Clinical guide for the suspicion and early diagnosis of tumors of the central nervous system.
- Walker D, Wilne S, Grundy R, Kennedy C, Neil, Dickson A, et al. A new clinical guideline from the Royal College of Paediatrics and Child Health with a national awareness campaign accelerates brain tumor diagnosis in UK children – “headSmart: Be Brain Tumor Aware.” Neuro Oncol. 2016; 18: 445-54.
Very interesting. Strategy launched in 2011 in the United Kingdom, aimed at healthcare professionals and the general public, with the aim of reducing the time interval from the onset of symptoms to the diagnosis of a CNS tumor in pediatric patients.
| Clinical case |
|
7-year-old patient who presents with a 3-day course of frontal, evening headache, as well as progressive 2-week loss of vision in the left eye. Personal history Normal course pregnancy. Vaginal delivery. Normal neonatal period. Normal psychomotor development. Up-to-date vaccination schedule. Family background Mother: 32 years old, healthy, gestations: 1/miscarriages: 0/life births: 1. Father: 38 years old, healthy. Physical examination HR: 90 bpm. RR: 18 rpm. BP: 100/60 mmHg. Sat: 97%. Height: 122 cm (50th centile). Weight: 24 kg (50th centile). Good general condition. Well hydrated and perfused. No rashes or petechiae. No significant lymphadenopathies. Cardio-pulmonary auscultation: normal. Abdomen: soft, non-tender, no masses or megalies. Neurological: Glasgow 15, with normal cranial nerves, tone, strength, sensitivity, tendon reflexes and gait. No dysmetria or dysdiadochokinesia. Ophthalmological examination: left eye with decreased visual acuity, signs of papillary atrophy, as well as left temporal hemianopsia. Complementary tests Normal full blood count, biochemistry and coagulation. A brain MRI was performed which evidenced a midline, suprasellar, multicystic, heterogeneous mass measuring 40 x 36 x 39 mm (Fig. 7), with intense and heterogeneous enhancement following contrast administration (Fig. 8). Normal ventricular system, without signs of hydrocephalus. Figure 7. Heterogeneous midline, suprasellar, multicystic mass measuring 40 x 36 x 39 mm. Figure 8. Midline mass with intense and heterogeneous enhancement following contrast administration. Diagnosis Craniopharyngioma (adamantinomatous pattern).
|
 How to suspect cancer in Primary Care
How to suspect cancer in Primary Care