|
| Topics on Continuous Training |
B. Rosich del Cacho*, Y. Mozo del Castillo**
*Consultant physician, Pediatrics Service, Hospital Universitario Joan XXIII, Tarragona. **Consultant physician, Pediatric Hemato-Oncology and Hematopoietic Stem Cell Transplantation Service, Hospital Universitario La Paz, Madrid
| Abstract
Anemia is defined as a reduction in hemoglobin concentration below normal levels for age, gender and ethnicity. It is the result of an imbalance between the production and destruction of red blood cells, which characterizes or accompanies various conditions. It is the most common hematological abnormality in childhood, the main cause of which is iron deficiency. Clinical manifestations are often nonspecific. Diagnosis begins with a full blood count, peripheral blood smear and biochemical parameters of hemolysis and iron metabolism. The overall diagnostic approach of the child with anemia is here reviewed, and an algorithm is proposed based on basic hematological data. Finally, a brief list of references is provided. |
Key words: Anemia; Child; Infant; Classification; Diagnosis.
Palabras clave: Palabras clave: Anemia; Niño; Lactante; Clasificación; Diagnóstico.
Pediatr Integral 2021; XXV (5): 214 – 221
 |
 |
Anemia. Classification and diagnosis
Introduction
Anemia is defined as a reduction in the concentration of hemoglobin or hematocrit, the normal levels of which depend on age, sex, and ethnicity. Iron deficiency anemia is the most prevalent hematologic disorder of childhood.
In this article, the general concepts of anemia in childhood and its diagnostic approach are reviewed. Iron deficiency anemia (the most common cause of anemia in the pediatric age) and hemolytic anemia are specifically discussed in other articles.
Definition(1,2)
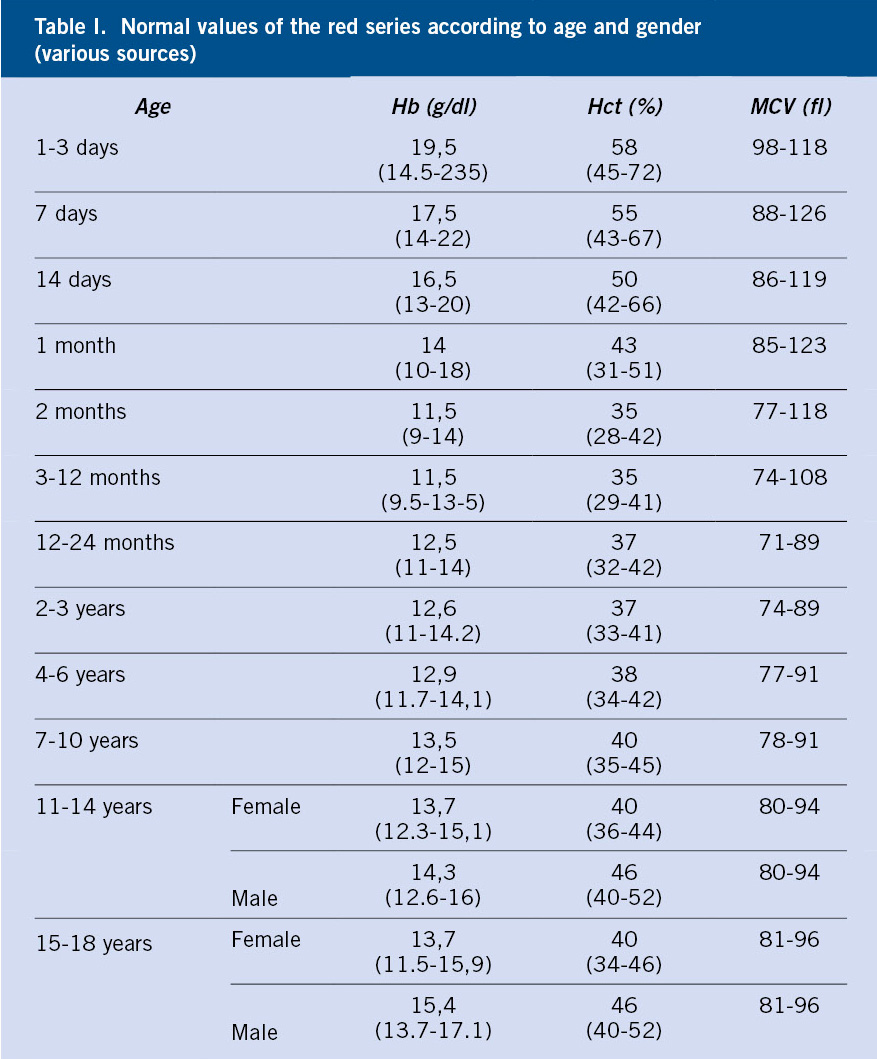
The word anemia is of Greek origin, meaning “without blood.” It is defined as the reduction in the concentration of hemoglobin (Hb), erythrocyte mass or hematocrit in peripheral blood below 2 standard deviations (-2 SD) for the age, sex and ethnicity of the patient (Table I).
• Hemoglobin (Hb): complex protein made up of heme groups containing iron and a protein portion, globin. The concentration of this erythrocyte pigment is presented in grams (g) per 100 ml (dl) of whole blood.
• Hematocrit (Hct): fraction of the volume of erythrocyte mass with respect to total blood volume. It is expressed as a percentage (%).
Epidemiology(2-4,7)
Anemia is the most common hematological disorder in childhood. In 2008, the World Health Organization (WHO) published the results of a survey of 192 member states, establishing the following Hb thresholds according to age group:
• 11 g/dl in children 0.5-4.99 years-old (preschool age).
• 11.5 g/dl between 5-11.99 years of age.
• 12 g/dl between ages 12-14.99 years.
The global prevalence of anemia was 47.4% ([95% CI] 45.7-49.1) in preschool-age children and 25.4% ([95% CI] 19.9-30.9) in school-age children. The prevalence in preschool children varied widely by country, led by countries in South America and Africa. This is because iron deficiency accounts for 50% of this prevalence and it is closely linked to nutritional deficiencies, therefore, to the social and development conditions in these countries.
In addition to acknowledging iron deficiency as the most common cause of anemia in pediatric age worldwide, we must take into account the factors and causes of this disorder that influence its prevalence:
• Age: Hb and Hct counts vary throughout childhood (Table I), as well as the causes of anemia differ according to the age of the patients:
– Birth-3 months: Hb achieves maximum concentrations (16.5-18.5 g/dl) in the newborn and drops to 9-10 g/dl between 6-9 weeks of life, as a consequence of increased oxygenation of tissues and a drastic decrease in erythropoiesis; in what is referred to as “physiological anemia of infancy”. Any anemia in this age group that differs from the characteristics of physiological anemia (Hb <9 g/dl, anemia prior to 1 month of age, or signs of hemolysis) will require further study.
– 3-6 months: iron deficiency is rare during this period, and hemoglobinopathies must be ruled out.
– 6 months-adolescence: there are differences in Hb counts according to age and sex (Table I). During this entire stage, the main cause of anemia is iron deficiency.
• Sex: starting at puberty, testosterone secretion induces an increase in erythrocyte mass, which is why the normal level of Hb is higher in men than in women. On the other hand, some hereditary anemias are X-linked, and hence, being more frequent in men (eg, glucose-6-phosphate dehydrogenase (G6PDH) deficiency and sideroblastic anemia).
• Race and ethnicity: normal Hb levels are observed with approximately 0.5 g/dl less in black children compared to those observed in Caucasians or Asians. HbS and HbC are more common in black and Hispanic populations. Furthermore, within the same country there are areas with a higher prevalence of hemoglobinopathies, endocellular parasites, such as malaria and infestation with intestinal parasites that impact on the prevalence of anemia. Thus, thalassemic syndromes are more prevalent on the Mediterranean coast, a large part of Africa, the Middle East, the Indian subcontinent, and Southeast Asia; conversely, G6PDH deficiency is observed, predominantly, in malaria endemic areas, since it seems to be a protective factor against this infection (a higher prevalence is found among: Kurdish Jews, Sardinian, Nigerian, African-American, Filipino and Greeks).
• Height above sea level: the higher above the sea level, the higher the Hb count, since lower oxygen content in the air results in a stimulus for hematopoiesis.
Pathophysiology(2,5)
Anemia is the result of the imbalance between production and loss of red blood cells. The redistribution of blood, the stimulation of erythropoiesis and the decrease in the affinity of Hb for O2 are compensatory mechanisms.
Erythropoiesis mainly takes place in the bone marrow during postnatal and adult life (in the fetal period and up to 6 months of extrauterine life, the endodermal sinus also participates, where it begins at 3-4 weeks of gestation, and later on in the liver). Various regulatory factors (being blood oxygen saturation the main one) act on the peritubular cells of the kidneys involved in the synthesis of erythropoietin (EPO), a hormone that acts on the hematopoietic precursors of the bone marrow, which finally give rise to mature red blood cells. During this complex process of differentiation and maturation leading to the production of the mature erythrocyte, the participation of different molecules, growth factors (G and GM-CSF), trace elements (such as iron, essential for the elaboration of the heme group of Hb, copper and zinc) and cytokines (IL 1, 3, 4, 6, 9 and 11).
Mature erythrocytes are shaped like a biconcave disc, they are filled with Hb in the inside and are devoid of mitochondria or other organelles. Hb is composed of 4 globin subunits and heme groups, and it is involved in the exchange of oxygen and carbon dioxide throughout the body.
After erythrocytes have been in circulation for a long period (half-life 120 days), they are taken up and destroyed by the reticuloendothelial system of the spleen. To maintain normal Hb levels, there must be a balance between the continued loss of senescent red cells and erythropoiesis in the bone marrow. Therefore, anemia is the result of the imbalance between production (decreased) and destruction or loss of red blood cells (increased).
There are different compensation mechanisms as an adaptive response to the situation of anemia:
• Redistribution of blood flow: this guarantees the oxygenation of vital organs (brain and myocardium), with the consequent vasoconstriction of less needy areas, such as skin and kidney.
• Stimulation of erythropoiesis: this is mediated by an increase in EPO synthesis, the main trigger of which is tissue hypoxia. This mechanism is only effective if the bone marrow is able to respond with increased production of red blood cells and the consequent increase in reticulocytes in peripheral blood.
• Increased ability of Hb to deliver oxygen to tissues: through an increase in the concentration of 2,3-diphosphoglycerate, which decreases the affinity of Hb for O2 and favors oxygenation of the tissues.
Understanding all of the above will help to understand the clinical manifestations and the pathophysiological classification of anemia, which are explained in the following sections.
Clinical manifestations(4,6)
Anemia can have both nonspecific clinical manifestations as well as guiding signs and symptoms for the etiological diagnosis.
The clinical picture of the anemic syndrome has common manifestations determined by: adaptation mechanisms, age of onset, underlying disease and type of onset (acute or chronic):
• Pale skin and mucous membranes: direct consequence of the decrease in Hb and the accompanying peripheral vasoconstriction. Sometimes pallor may not be evident until the Hb level falls below 8 g/dl and can be difficult to identify depending on the pigmentation of the skin.
• Cardiocirculatory symptoms and signs (palpitations, tachycardia, systolic murmur, exertional dyspnea and tachypnea): in general, they are due to the onset of compensatory mechanisms due to the decrease in blood volume, and become more evident the greater the degree of anemia and the speed of its establishment.
• General symptoms (headache, irritability, mood swings, asthenia, anorexia): due to tissue hypoxia. In chronic anemia, there may also be an impact on diverse organs, leading to: neurological dysfunction (impaired psychomotor development, learning difficulty), delayed puberty, osteopenia, cardiological abnormalities (left ventricular hypertrophy that can lead to heart failure), etc.
• Accompanying symptoms: derived from the causes and pathogenic mechanisms involved, such as:
– Hemolytic anemia: jaundice, choluria, abdominal pain, splenomegaly (due to the role of the spleen in the destruction of erythrocytes) or hepatosplenomegaly (because of extramedullary erythropoiesis), gallstones and bone abnormalities (due to extramedullary erythropoiesis).
– Deficiency anemia: trophic disorders of the skin and mucous membranes.
– Anemia of central origin (medullary): bleeding and infections if associated with thrombopenia and leukopenia.
Diagnosis(2,7-9)
Adequate history-taking, physical examination, full blood count with red cell indices, reticulocytes, peripheral blood smear and biochemistry, are cost-effective tools for the diagnostic approach of anemia.
Medical history
An adequate medical history is the starting point for the etiological diagnosis of anemia. In addition to noticing the age, gender, ethnicity and geographical origin of the patient, the following should be investigated:
• Symptoms (see previous section): beginning and speed of onset, tolerance, history of bleeding (digestive, menstrual, etc.), symptoms suggestive of hemolysis, etc.
• Neonatal history: gestational age, blood group, history of hospital admission for jaundice/anemia, neonatal screening results for endocrine-metabolic diseases (sickle cell anemia is included in Spain).
• Underlying pathology: previous episodes of anemia and treatment received, presence of coagulopathy, concomitant diseases (infectious and/or inflammatory), malabsorptive problems (eg, celiac disease).
• Family history: the former existence of: anemia, jaundice, gallstones, splenomegaly or the need for cholecystectomy in family members, can guide the diagnosis of hereditary hemolytic anemia.
• Diet: to be queried in order to consider possible nutritional deficiencies (iron, vitamin B12 and folic acid). It is important to document the type of lactation (breastfeeding/artificial formula), the amount and the possibility of supplementation/fortification. The presence of pica can guide towards a deficit of nutrients. To consider that the intake of certain foods (eg: fava beans) can trigger hemolytic crises in G6PDH deficiency.
• Exposure to drugs/toxins: medications (antibiotics, anti-inflammatory drugs, anticonvulsants), herbs, homeopathic products, drinking water containing nitrates, oxidants or products with lead.
Physical examination
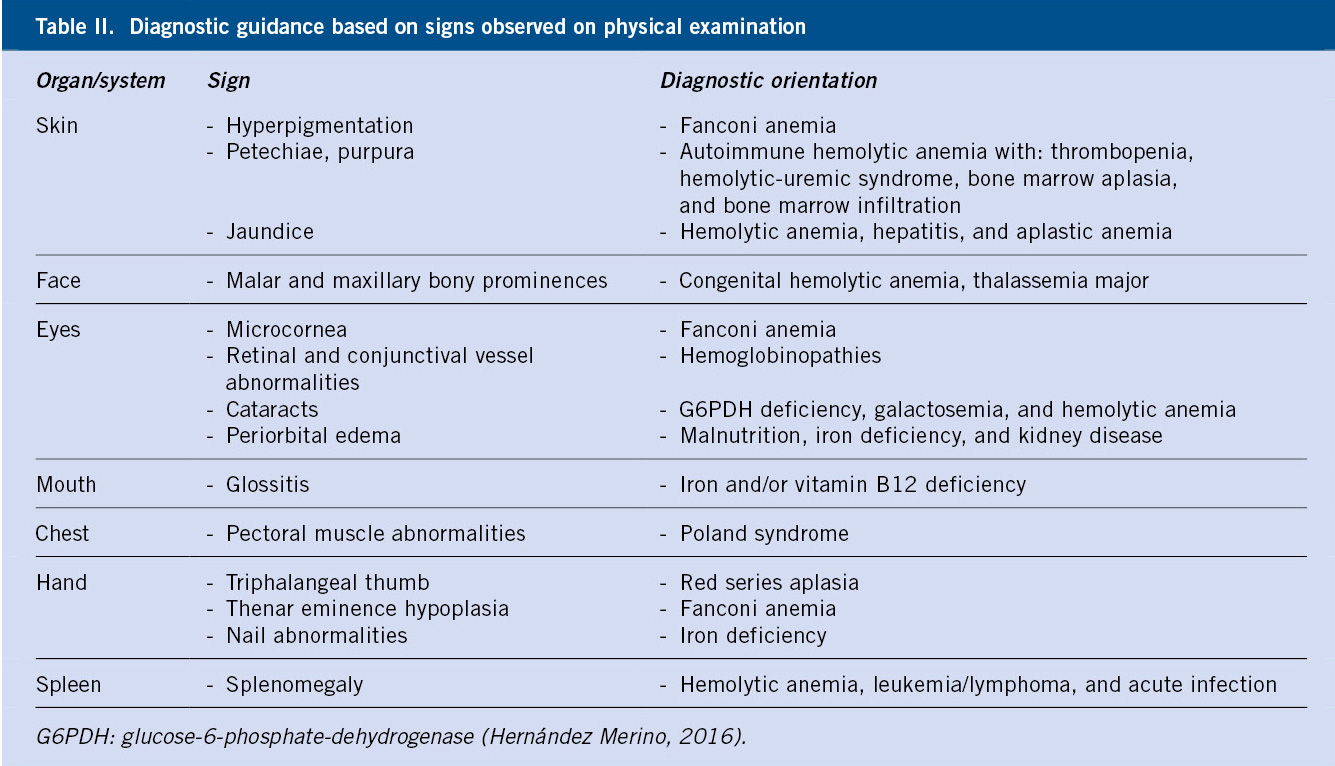
Special attention should be paid to: skin, eyes, mouth, face, chest, hands and abdomen. Skin paleness is a specific but poorly sensitive finding; and so is tachycardia, as a manifestation of severity. Jaundice and hepatosplenomegaly, characteristic of hemolysis, are also specific data, but with relatively low sensitivity.
Table II shows certain physical signs which can guide towards a specific cause of anemia.
Laboratory evaluations
Work-up tests should begin with a full blood count with red cell indices and a thorough examination of the peripheral blood smear. In addition, first-level studies require: reticulocyte count, basic biochemistry and study of iron metabolism.
In any study of anemia, we must take into account the following parameters of the full blood count, which will aid in the classification of anemia and consequently in its etiological diagnosis:
• Mean corpuscular volume (MCV): this is the average size (fl) of the red blood cells. Depending on this value, the morphological classification of anemia will be carried out (see next section).
• Red cell distribution width (RDW): this informs of the coexistence of red cell populations of different sizes. This parameter helps to distinguish between iron deficiency and thalassemia, since in the former it is usually high, due to the different distribution of Hb depending on the iron availability at each moment; whilst in thalassemia, RDW is usually normal (although it may be elevated), because the Hb distribution is uniform.
• Reticulocyte Production Index (RPI): reticulocytes report on the regenerative capacity of the bone marrow, so this parameter will allow a pathophysiological classification of anemia (see next section). Normal values of RPI range between 2 and 3. The correct interpretation of the reticulocyte number requires the adjustment of the crude number (%) according to the real number of red cells of each patient, using the formula shown in figure 1.
Figure 1. Formula for adjusting the reticulocyte count.
• Peripheral blood smear examination: this must be performed by an experienced hematologist. The size and morphology of the red blood cells can be essential to identify disorders such as: sickle cell disease (sickle cells), spherocytosis (spherocytes), hemoglobinopathies (target cells), hemolysis (Heinz bodies), etc. Additionally, the presence of other cytopenias or leukocytosis with immature forms can point toward certain etiologies (infections, medullary aplasia/hypoplasia, infiltration of the bone marrow due to leukemia/lymphoma, etc.).
• Iron metabolism: ferritin is the most useful parameter to measure iron stores; values below 15mcg/l are indicative of iron deficiency. However, its usefulness is limited by its role as an acute phase reactant, increasing with inflammation/infection and tissue destruction.
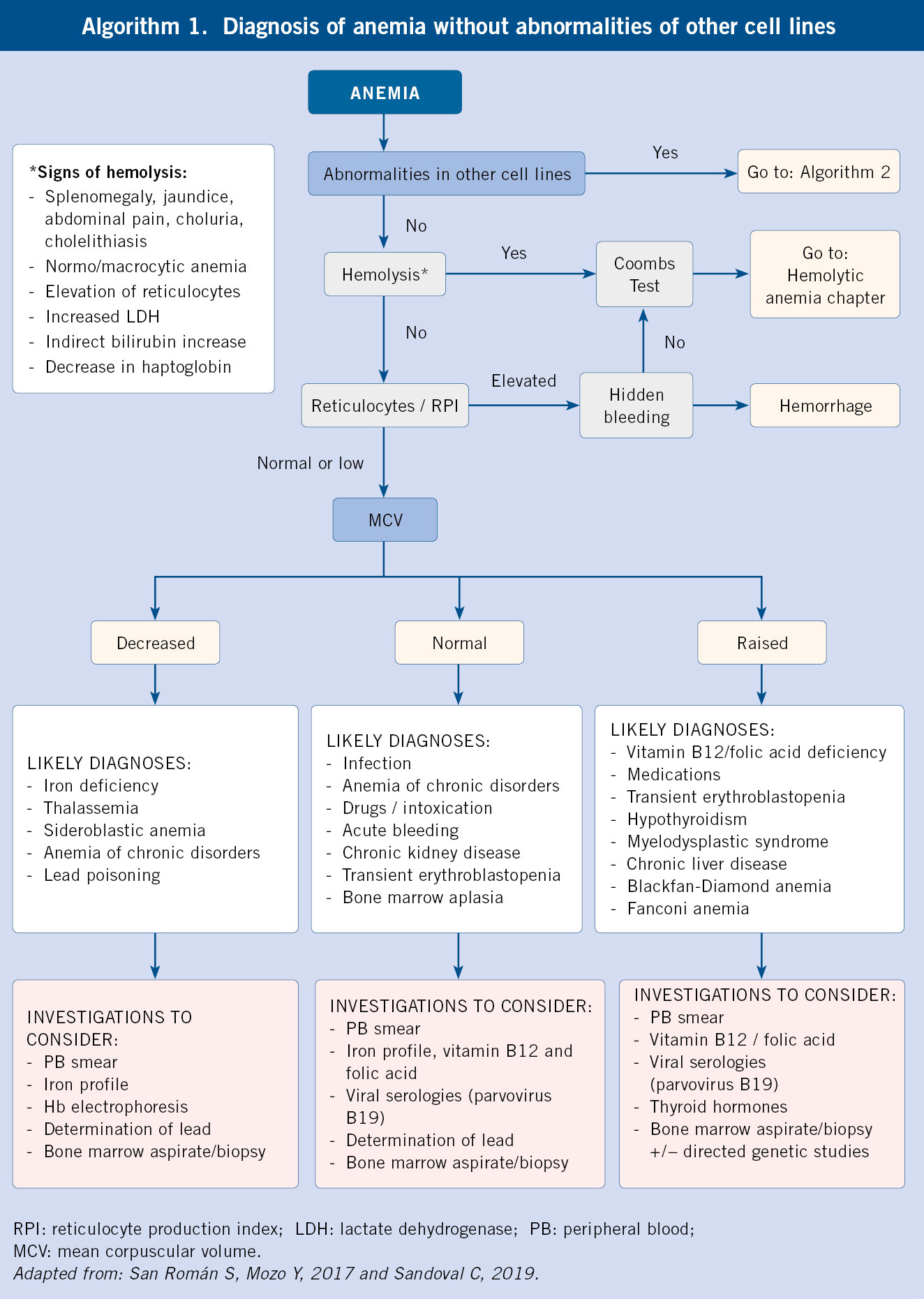
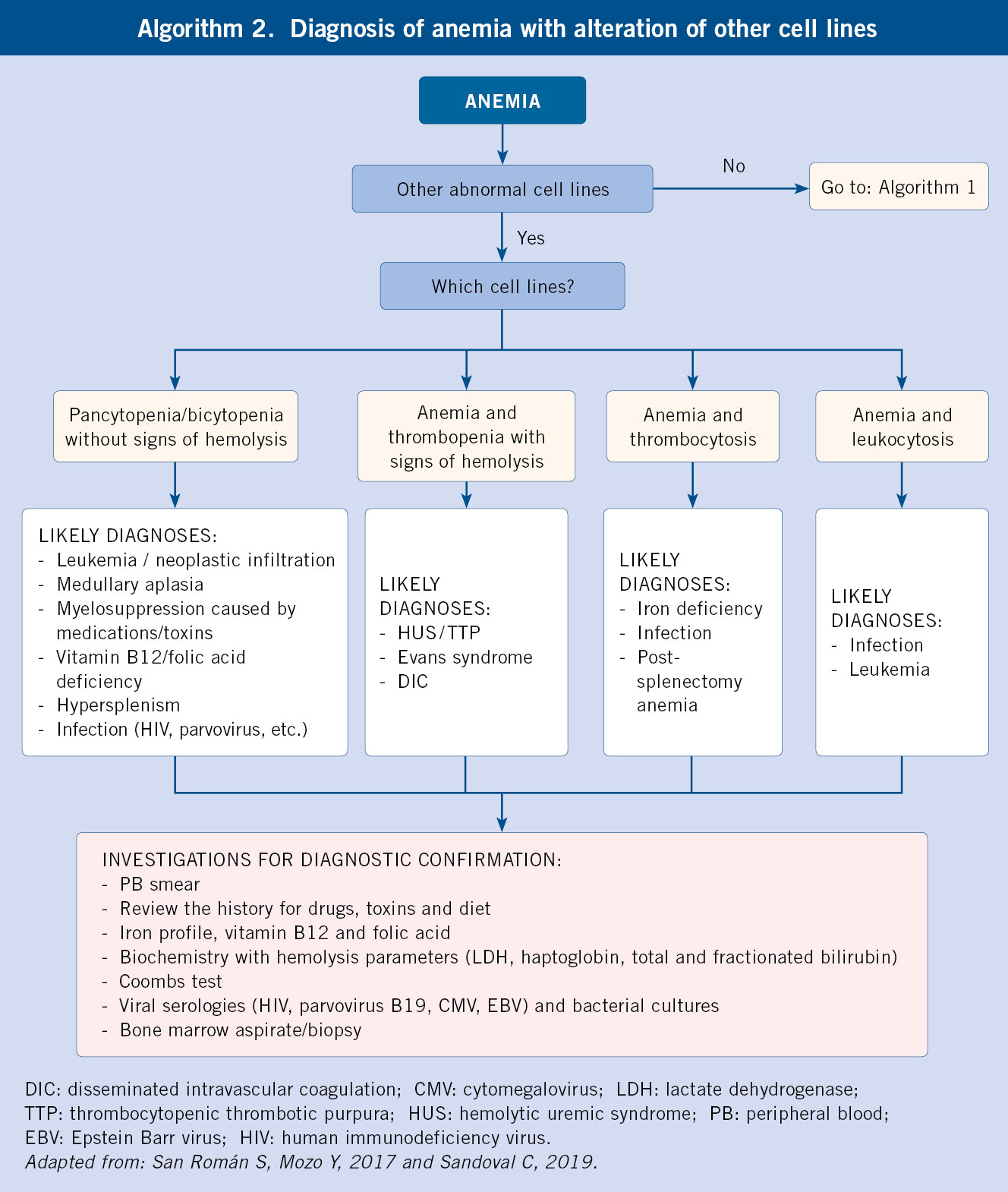
According to the previous findings, we will have to expand the investigations with second level studies (Diagnostic algorithms, algorithms 1 and 2):
– If reticulocytosis and data suggestive of hemolysis are present, the existence of hemolytic anemia should be ruled out and the following should be requested: haptoglobin, Coombs test to determine autoimmunity, and if negative, hemoglobin electrophoresis to rule out hemoglobinopathy (considering that HbF is predominant within the first months of life, and its decrease is slower in sickle cell disease, where it persists in variable amounts) and/or quantification of enzymes (G6PDH, pyruvate-kinase) to study enzymopathies, and/or membrane studies (osmotic fragility, cytometry) for membranopathies.
– If macrocytosis (elevated MCV) is found: there may be a deficiency cause (vitamin B12, folic acid) or hypothyroidism (request TSH and FT4), but it could also be a central maturational disorder (bone marrow aspirate/biopsy must be performed).
– If there is reticulocytopenia and parvovirus B19 infection has been ruled out, a bone marrow aspirate/biopsy must be carried out to assess anemia of central origin.
Classification of anemia(2,7-9)
Morphological and pathophysiological classifications of anemia are complementary and required for the etiological diagnosis approach.
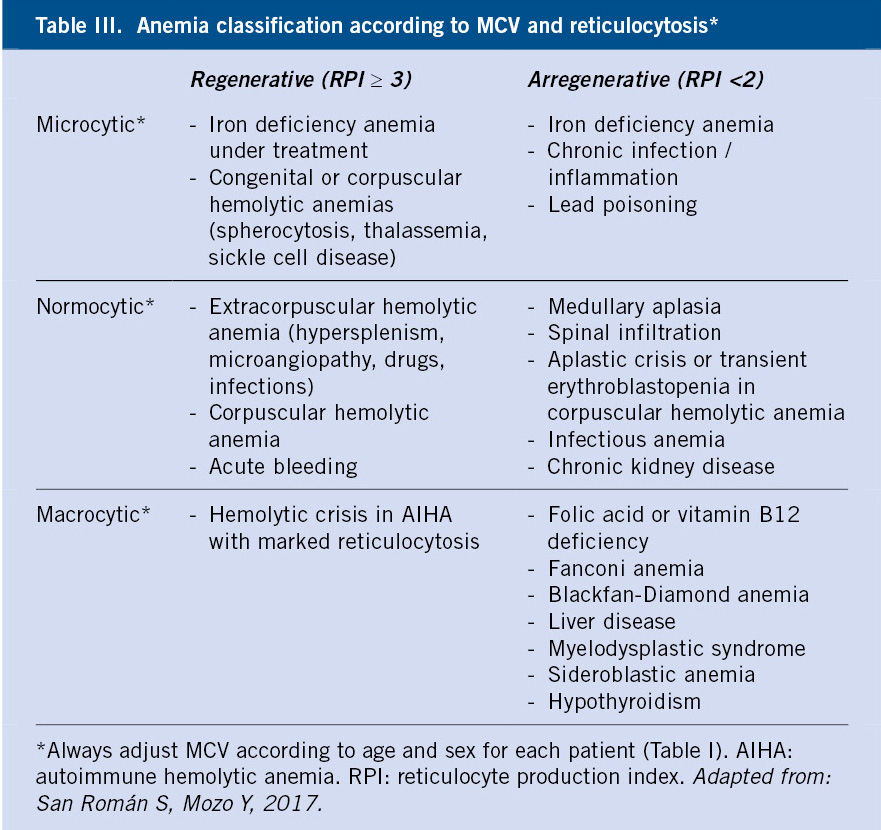
Following the results obtained in the first-level investigations, the morphological and pathophysiological classifications of anemia will be carried out, which will lead to the determination of the etiology after conducting specific additional work-up (Table III and Algorithms 1 and 2).
• Morphological classification: based on the MCV (Table I).
– Microcytic: MCV ≤ 2.5 percentile for age, sex, and ethnicity. In childhood, these are iron deficiency anemia and thalassemia par excellence.
– Normocytic: Normal MCV (between 2.5 and 97.5 percentiles) for age, sex and ethnicity. This can be due to: blood loss, chronic disorders, infections or it can be the initial stage of micro or macrocytic anemia.
– Macrocytic: MCV ≥ 97.5 percentile for age, sex, and ethnicity. In childhood the most common ones are due to vitamin B12 and/or folic deficiency or exposure to certain drugs (eg, anticonvulsants and immunosuppressants). Macrocytosis can also be found in acute regenerative anemia, due to the presence of reticulocytosis, and in maturation disorders of the bone marrow (myelodysplasia and aplasia).
• Pathophysiological classification: based on the medullary regenerative capacity, determined by the RPI.
– Regenerative: there is an elevated reticulocyte response (reticulocytes >3% or RPI ≥3). Examples of this include: hemolytic anemia and anemia secondary to hemorrhage.
– A/hyporegenerative: normal or low reticulocyte response for the degree of anemia (reticulocytes <1-1.5% or RPI <2). It translates the existence of a hypo/inactive bone marrow as a result of various reasons: deficiency of substrates (iron, folic acid, vitamin B12), tumor medullary infiltration, infections, deposit diseases (Nieman-Pick, Gaucher…) or congenital or acquired aplasia.
Role of the Primary Care pediatrician
• From the Primary Care setting, full blood count with erythrocyte indices, reticulocytes, biochemistry with hemolysis parameters and iron profile, can be requested, which provide the diagnostic approach of most anemia cases in the pediatric age.
• In our environment, the anemia cases that the Primary Care pediatrician can most frequently find are: iron deficiency anemia, physiological anemia of infancy, β-heterozygous thalassemia or thalassemic trait, anemia of chronic disorders, anemia due to hemorrhage and, currently also, sickle cell anemia.
• In general terms, any anemia of the following characteristics: combined with another cytopenia, hemoglobinopathies, hemolytic anemia, aregenerative anemia, those that do not respond to treatment, and whenever there is any other warning sign, is susceptible to referral to specialized care.
Bibliography
The asterisks show the interest of the article in the opinion of the authors.
1. Glader B. Anemia. In: Kliegman RM, Behrman RE, Jenson HB, Stanton BF, ed. Nelson. Textbook of Pediatrics. 18th ed., Barcelona. Elsevier España; 2009. p. 2003-6.
2. Hernández Merino A. Anemias en la infancia y adolescencia; clasificación y diagnóstico. Pediatr Integral. 2016; XX(5): 287-96.
3. Word Health Organization. Worldwide prevalence of anemia 1993-2005: WHO global database on anemia. Geneva: World Health Organization; 2008.
4.** Allali S, Brousse V, Sacri AS, Chalumeau M, de Montalembert M. Anemia in children: prevalence, causes, diagnostic work-up, and long-term consequences. Expert Rev Hematol. 2017; 10: 1023-8.
5.** Arrizabalaga B, González FA, Remacha. Eritropatología. Edición Ambos Marketing Services. Barcelona. 2017.
6.** Prudencio García-Paje M. Aproximación diagnóstica al paciente con anemia. In: Madero L, Lassaletta A, Sevilla J, ed. Hematología y Oncología Pediátricas. 3ª edición, Madrid. Ergon; 2015. p. 81-6.
7.*** Sandoval C. Approach to the child with anemia (Literature review: September 2020. Last update: June 20, 2019). Available at: www.uptodate.com.
8.** San Román Pacheco S, Mozo del Castillo Y. Síndrome anémico. In: Guerrero-Fdez. J, Cartón Sánchez A, Barreda Bonis A, Menéndez Suso J, Ruiz Domínguez J, ed. Manual de Diagnóstico y Terapéutica en Pediatría. 6ª edición, Madrid. Editorial Panamericana; 2017. p. 1117-30.
9. Buttarello M. Laboratory diagnosis of anemia: are the old and new red cell parameters useful in classification and treatment, how? Int J Lab Hematol. 2016; 38: 123-32.
10. Sevilla Navarro J. Abordaje de la anemia microcítica; nuevas herramientas diagnósticas. In: AEPap, ed. 7º Curso de Actualización en Pediatría 2010. Madrid: Exlibris Ediciones; 2010. p. 239-9.
Recommended bibliography
– Allali S, Brousse V, Sacri AS, Chalumeau M, de Montalembert M. Anemia in children: prevalence, causes, diagnostic work-up, and long-term consequences. Expert Rev Hematol. 2017; 10: 1023-8.
This article summarizes in a practical way, the causes of anemia in Pediatrics and the investigations necessary for its diagnosis, highlighting the comorbidity that this pathology implies in the long term.
– Arrizabalaga B, González FA, Remacha. Eritropatología. Edición Ambos Marketing Services. Barcelona. 2017.
General hematology chapter, suitable for the study of hematopoiesis and the pathophysiology of anemia.
– Prudencio García-Paje M. Aproximación diagnóstica al paciente con anemia. In: Madero L, Lassaletta A, Sevilla J, ed. Hematología y Oncología Pediátricas. 3ª edición, Madrid. Ergon; 2015. p. 81-6.
Chapter where a general presentation is made on anemia in Pediatrics, useful to make a global approach to the subject. In the same book, there are other specific chapters describing the different types of anemia in greater depth.
– Sandoval C. Approach to the child with anemia (Literature review: September 2020. Last update: June 20, 2019). Available at: www.uptodate.com.
This frequently updated source of evidence-based medicine offers a comprehensive review of childhood anemia, presented in a clear educational structure.
– San Román Pacheco S, Mozo del Castillo Y. Síndrome anémico. In: Guerrero-Fdez. J, Cartón Sánchez A, Barreda Bonis A, Menéndez Suso J, Ruiz Domínguez J, ed. Manual de Diagnóstico y Terapéutica en Pediatría. 6ª edición, Madrid. Editorial Panamericana; 2017. p. 1117-30.
Presentation of the topic of anemia in childhood, with practical tables and algorithms.
| Clinical case |
|
Presentation A 7-year-old girl is seen in clinic because of progressive paleness and a one-month clinical picture of asthenia, initially attributed by the family to various infectious processes of probable viral etiology (gastroenteritis, upper respiratory tract catarrh and otitis), without any of them requiring admission or antibiotic therapy. Her physical activity remains intact. She has not had: fever, sweating, anorexia, weight loss or musculoskeletal pain. She had taken mebendazole in the month prior to the consultation as treatment for oxyuriasis, and ibuprofen occasionally. Her pediatrician had requested a test where Hb of 8 g/dl stands out. Deficiency and/or parainfectious etiology was suspected, and iron therapy was prescribed. She attends the clinic today after two weeks of starting treatment for clinical and analytical control. Personal history • Up-to-date vaccination, according to her region´s (autonomous community) immunization schedule. • No known allergies. • No medical-surgical history of interest. • Pets: a dog who has lived at home for 10 years, correctly vaccinated. • She lives in an urban area. No recent trips. • Exercise: swimming. Family background • Parents and a 10-year-old brother, all healthy. • Maternal grandmother: deficiency anemia. • No other background of interest. Physical examination Weight: 20 kg. Temperature: 36.5°C. Respiratory rate: 22 rpm. Heart rate: 105 bpm. Systolic/diastolic blood pressure: 115/65 mmHg. Good general condition. Marked skin and mucosal paleness. No rashes or petechiae. No respiratory distress. Good peripheral perfusion, capillary filling time <2 seconds, peripheral pulses are present and symmetrical. Cardiac auscultation: rhythmic sounds, systolic murmur II/VI. Lung auscultation: normal. Normal oropharynx. Normal bilateral otoscopy. No pathologic lymphadenopathies. Soft, non-tender abdomen, without masses or organ enlargement. Preserved peristalsis. No signs of peritoneal irritation. Glasgow coma scale 15/15, collaborating, negative meningeal signs, no neurological focus. Work-up tests • Full blood count: Hb: 6.5 g/dl; MCV: 89 fl; leukocytes: 4.76×109/l; neutrophils: 0.98×109/l; lymphocytes: 3.61×109/l; monocytes: 0.16×109/l; platelets: 120×109/l; MPV: 10 fl. Reticulocytes: 21×109/l (0.7%). • Biochemistry: glucose: 80 mg/dl; urea: 25 mg/dl; creatinine: 0.4 mg/dl; sodium: 140 mEq/l; potassium: 4.1 mEq/l; chloride: 105 mEq/l; GOT: 29 IU/l; GPT: 21 IU/l; total bilirubin: 0.4 mg/dl; LDH: 206 IU/l; phosphorus: 5.5 mg/dl; total calcium: 10 mg/dl.
|
 Anemia. Classification and diagnosis
Anemia. Classification and diagnosis