|
| Topics on Continuous Training |
J. Huerta Aragonés, C. Mata Fernández
Pediatric and Adolescent Hematology and Oncology Section. Pediatrics Service. Gregorio Marañón General University Hospital. Maternal and child Hospital. School of Medicine. Complutense University of Madrid. Gregorio Marañón Health Research Institute (IiSGM). CIBEREHD
| Abstract
Over the last several decades there has been a significant increase in the survival of most pediatric tumors, which is undoubtedly a success, but generates a new scenario with an increase in late side effects and premature mortality compared to the general population. Therefore, it is highly important to develop models for follow-up care of childhood cancer survivors, which allow fluid and efficient communication between healthcare professionals, individualized management for each patient, implementation of healthy lifestyle habits and early diagnosis protocols for relapses and late sequelae. Following this approach, adherence to follow-up will be increased and the severity of chronic toxicity will be reduced. There are national and international consensus guidelines of increasing quality, but with a high degree of heterogeneity between them. In this article reference will be made to these follow-up guidelines, highlighting the specific late side effects on each organ system, their prompt recognition and recommendations for longterm management. |
| Resumen
En las últimas décadas, se ha experimentado un notable incremento en la supervivencia de los tumores diagnosticados en edad pediátrica, lo cual supone indudablemente un éxito de la medicina, pero genera un nuevo escenario, con un aumento de los efectos secundarios tardíos y de la mortalidad prematura respecto a la población general. Es de vital importancia, por tanto, el desarrollo de modelos de seguimiento del paciente superviviente, que permitan una comunicación fluida y eficiente entre profesionales sanitarios, un manejo individualizado para cada paciente, la implementación de hábitos de vida saludables y de protocolos de diagnóstico precoz, de recaídas y de secuelas tardías. De esta forma, se aumentará la adherencia al seguimiento y se reducirá la gravedad de la toxicidad crónica. |
Key words: Supervivientes de cáncer infantil; Efectos secundarios tardíos; Toxicidad a largo plazo; Segundas neoplasias.
Palabras clave: Childhood cancer survivors; Late side effects; Long-term toxicity; Second malignancies.
Pediatr Integral 2021; XXV (7): 372 – 385
Follow-up of childhood cancer in Primary Care. How to detect late effects
Introduction
Cancer survival rates in the pediatric age have notably increased in recent decades, where prevention as well as early diagnosis of late sequelae strategies have become essential.
The number of childhood cancer survivors has increased significantly in the last 40 years, increasing from 58% survival between 1975-1977 to 83% between 2008-2014(1). In Spain, a 23% increase in survival was observed (from 54 to 77%, from 1980-1984 to 2000-2004), with a 50% decrease in the risk of death in that period (https://www.uv.es/rnti/cifrasCancer.html). In Europe, 1 in 500-600 children will develop a malignancy before the age of 15 and it is estimated that, in the next few years, one in 450 young adults will be a childhood cancer survivor in Europe(2). In contrast to this great advance, there is a high prevalence of morbidity in the form of late side effects. The Children’s Oncology Group (COG) in a childhood cancer survival study observed the existence of at least one chronic problem in the second decade of life in almost 60% of survivors and more than 30% of severe chronic sequelae 30 years after diagnosis(3). The St. Jude Lifetime Cohort Study published a study in which it was observed that at 45 years of age, 95.2% of the survivors reported at least one chronic health condition, being it serious, disabling or life threatening in 80% of the cases (twice that of the general population at that age)(4).
Cancer treatments predispose recipients to excess morbidity and premature mortality compared to the general population. The risk is directly proportional to the intensity of the treatment to achieve the cure of the disease, being more burdensome in the case of multimodal therapy and in patients who have received several treatments due to relapse. Age is a determining factor, the younger the more toxic effects on linear growth, skeletal maturation, intellectual functionality, sexual development and organic functionality.
Although these are worrisome data, the impact of these late effects can be modified through early detection and adequate management of the pathologies, as well as, prevented in part through a modification of lifestyle habits(1). Likewise, it has become a generalized trend of current protocols to seek, in cases where a good long-term survival result has been achieved, to reduce acute and chronic toxicity without detriment to survival (e.g., acute lymphoblastic leukemia, nephroblastoma, Hodgkin lymphoma, certain brain tumors…), in particular, reducing the use of radiotherapy and adjusting the intensity of treatment to the individual risk(5,6).
Reducing late effects and early detection of them, if they exist, has become a goal for these patients, in order to improve their quality of life. Follow-up and recommendations must be individualized according to the personal characteristics of each survivor, type of tumor, treatment received, age, comorbidities, lifestyle, and existence of dysmorphic syndromes or predisposition to cancer(1) (Fig. 1). Side effects increase with age, but can manifest throughout life, hence, an adequate monitoring based on recommendations and consensus guidelines, from pediatric age to adulthood is essential.
Figure 1. Lifestyle recommendations and health promotion.
Follow-up Models of Childhood Cancer Survivors
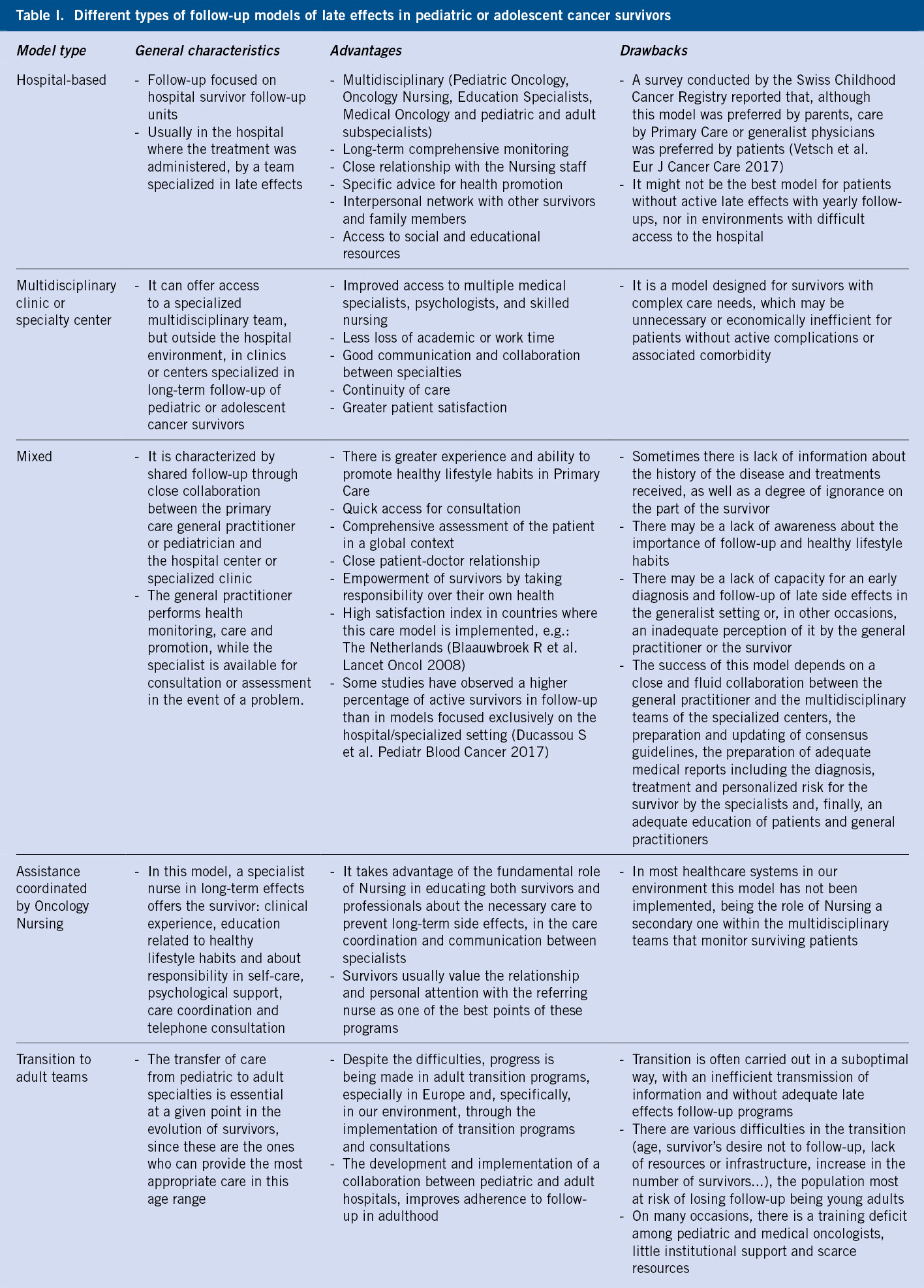
There is not a unique model for the monitoring of long-term side effects, and the superiority of one over the others has not been demonstrated by studies of adequate duration and methodology. Table I contains a comparison between the different models, their advantages and disadvantages(1).
In general, the most suitable option would be the one that best suits the individual characteristics of the patient. In our environment, joint work between healthcare professionals from the hospital and Primary Care setting is essential, as well as a progressive and structured transition to the adult care model. Information transfer is crucial, especially: previous medical history, dates of diagnosis and end of treatment, diagnostic tests at diagnosis and during follow-up, multimodal treatments received and their doses, complications related to them or to the disease itself, relapses and follow-up plan (Table II).
Producing detailed medical reports for the physicians who will provide ongoing care for survivors in Primary Care or during their adulthood is paramount and often one of the deficits of hospital care. In this sense, a good number of Pediatric and Adolescent Oncology and Hematology services are making efforts to implement specific follow-up consultations for the survivor and transition to adult care, paying special attention to the elaboration of “survivor’s passport”. There are tools promoted by the International Society of European Pediatric Oncology (SIOPe) (http://www.survivorshippassport.org/) and through European projects such as the PanCareSurPass project (Horizon 2020-EU.3.1.5 Framework Program, https://cordis.europa.eu/project/id/899999/es).
Follow-up recommendations
There are consensus guidelines for the follow-up of survivors from different international groups that, although heterogeneous and variable in the recommendations, result very useful.
In recent years, national and international guidelines have been developed for the monitoring of long-term side effects in survivors. In the United States, the Children’s Oncology Group (COG) periodically publishes the “Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers” (latest version: 5.0, October 2018). It is a guide based on the individual risk, tools for diagnosis and management of side effects. The current version is accessible through the website www.survivorshipguidelines.org(7). In Europe, three large groups have developed similar guidelines: the UKCCSG (United Kingdom Children’s Cancer Study Group) “Therapy Based Long-Term Follow Up Practice Statement”(8), the “Long Term Follow Up of Survivors of Childhood Cancer, A National Clinical Guideline” (Scottish Intercollegiate Guidelines Network, SIGN)(9) and the “Guidelines for follow-up after childhood cancer more than 5 years after diagnosis” (Late Effects Taskforce of the Dutch Childhood Oncology Group, DCOG LATER)(10). Each of these recommendations uses a different methodology and, at times, the recommendations are variable. With the aim of homogenizing them, the International Late Effects of Childhood Cancer Guideline Harmonization Group (IGHG) was created, however, given the complexity of the issue, for now, it has only prepared documents for the screening of breast cancer, cardiomyopathy, early ovarian failure, male gonadal toxicity, thyroid cancer and ototoxicity (www.ighg.org/guidelines/). The Spanish Society of Pediatric Hematology and Oncology (SEHOP) published the guide “Late effects in survivors of childhood cancer” in 2012, which constitutes a tool of indisputable value(11). The need to develop consensus guidelines is obvious, but the long-term cost-effectiveness of these recommendations remains unknown(1).
Late effects on organs and body systems
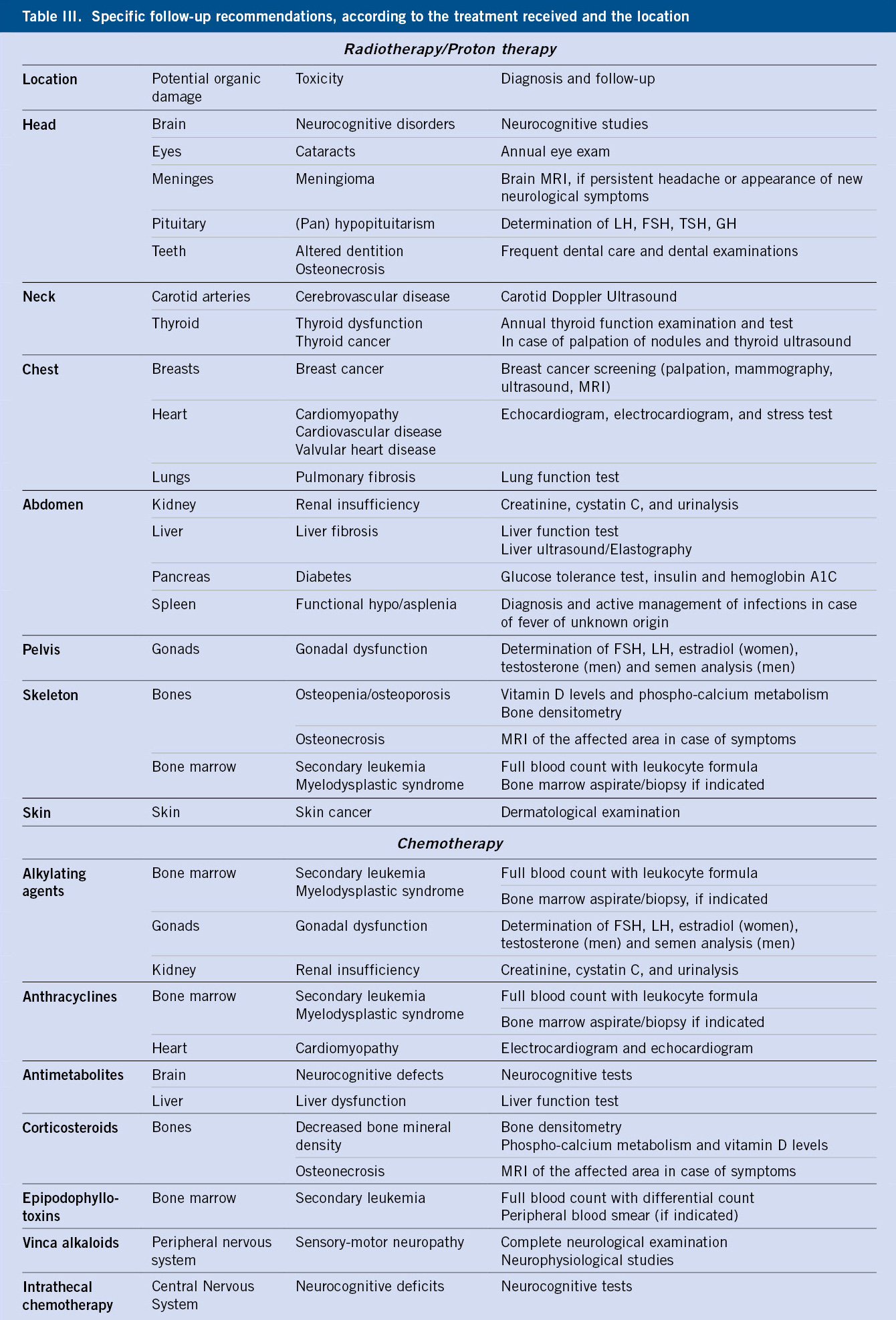
There are guidelines for side effects tailored to each specific treatment that, due to their breadth, far exceed the length of this article(7,8). Thus, we will focus on side effects by organs or systems. They are shown in table III in a very summarized manner.
Cardiology sequelae
Patients at greatest risk are those receiving high-dose anthracyclines, chest or mediastinal radiation therapy, or both; however, other conventional and targeted therapies also require vigilance.
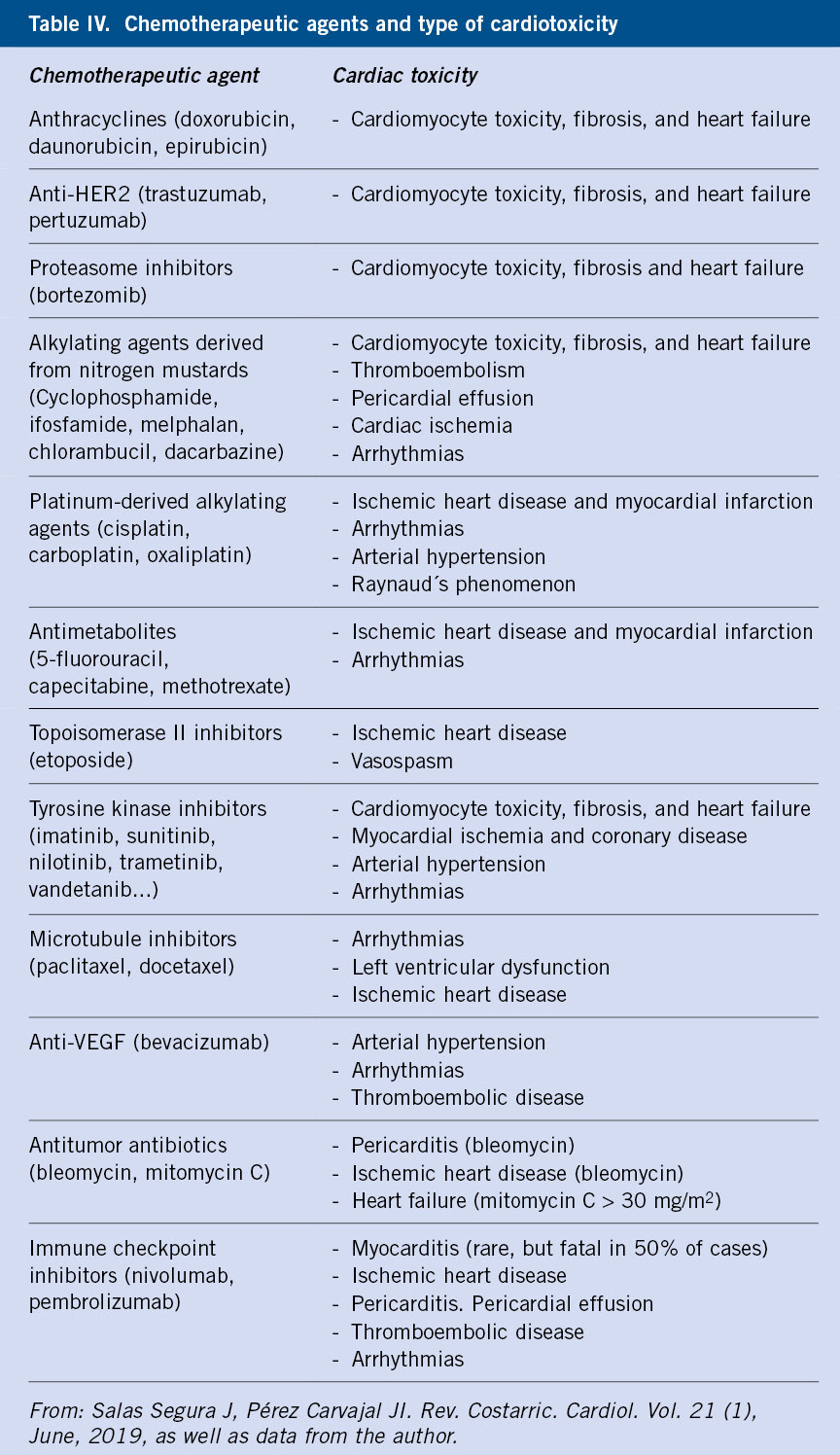
Cardiovascular complications are one of the main problems for survivors, with an increased risk of heart failure compared to the general population (15 times higher) and premature death from cardiac causes (7 times higher)(12). Anthracyclines, regardless of the type, produce direct and indirect toxicity (free radicals) on the myocyte. There is no safe dose if long-term normal cardiovascular status is the goal. Chronic toxicity manifests itself months or even many years later, consisting of dose-dependent non-ischemic degenerative dilated cardiomyopathy (more frequent than restrictive) due to irreversible myocardial damage, as well as arterial hypertension, ischemic heart disease, and heart failure. Serious arrhythmias, tachycardia, ventricular fibrillation and even second- and third-degree blocks may appear. The incidence of conventional cardiotoxicity is 7.5-10% 30 years after finishing treatment(12,13). Female sex, earlier age, higher cumulative dose (> 250 mg/m2), and concomitant cardiothoracic irradiation are associated with an increased risk of cardiotoxicity and greater severity. There are also other classic chemotherapy agents (cyclophosphamide, cytarabine, cisplatin, ifosfamide, paclitaxel, 5-fluorouracil…) that produce cardiac toxicity by other mechanisms(14). In recent years, other emerging therapies must also be considered, such as proteasome, HER2, VEGF and tyrosine kinase (TK) inhibitors, as well as immunotherapy, including CAR-T cell therapy and immune checkpoint inhibitors(15) (Table IV).
The development of cardioprotective molecules has been a desire over the last years. Dexrazoxane has been postulated as a strong candidate, as it improves the cardiotoxicity observed with anthracyclines. Its use has been limited by the suspicion that it could interfere with the efficacy of anthracyclines, as well as by a possible contribution to the development of secondary neoplasms. None of these aspects have been proven in recent studies, including a meta-analysis with a sample of 4,639 children treated with anthracyclines for different neoplasms (Shaikh F et al., J Natl Cancer Inst. 2016). The American Heart Association and the American Academy of Pediatrics recommend its use as a cardioprotective agent in protocols that use anthracyclines (the Children’s Oncology Group -COG- recommends it since 2015 in those protocols that involve doses ≥ 150 mg/m2 or cardiothoracic radiotherapy). In our setting, it is not routinely used in the clinical practice. The use of liposomal forms of anthracyclines and analogues (epirubicin, idarubicin, mitoxantrone) could improve long-term toxicity(14) and long-term (6-96 hours) dosing regimens are recommended instead of boluses. Drugs such as enalapril or phosphocreatine (Cheuk DKL et al. Cochrane Database Syst Rev. 2016), beta-blockers, statins or anti-aldosteronic agents have not been shown to have a protective effect.
The mediastinal/thoracic radiotherapy produces acute inflammation on the cardiomyocytes and generates hypercoagulability. Oxidative stress leads to chronic inflammation and subsequent fibrosis (Velásquez CA et al. Rev Colombiana Cardiol 2018). Risk factors include: high cumulative doses (> 30-35 Gy), high daily doses (> 2 Gy/session), age <50 years at the time of therapy, previous history of heart disease or cardiovascular risk factors, and concomitant cardiotoxic chemotherapy treatment(7). Toxicity manifests as: ventricular dysfunction, endothelial damage (increased vascular risk, coronary artery disease, degenerative aortic vascular disease or supra-aortic trunk disease, stroke), dilated or restrictive cardiomyopathy, chronic/constrictive pericarditis, degenerative valvular disease and arrhythmias.
In summary, high-risk patients are those who have received ≥ 250 mg/m2 of anthracyclines, > 35 Gy of chest radiation or ≥ 100 mg/m2 of anthracyclines + ≥ 15 Gy of irradiation(12). Surveillance in high-risk patients should begin no later than 2 years after the end of cardiotoxic treatment and should be repeated 5 years after diagnosis, continuing every 5 years or sooner if necessary.
The semiology to be monitored will be dyspnea, chest pain, palpitations, intolerance to exercise and activities of daily life. Routine cardiopulmonary auscultation is recommended, as well as ruling out the presence of carotid murmurs. Echocardiography is the main imaging test for diagnosis and follow-up, with magnetic resonance imaging being an interesting supplementation in selected cases (not as a screening technique)(16). The electrocardiogram is a tool for the diagnosis of arrhythmias and cardiomyopathy, being necessary, on occasions, the performance of stress tests and Holter tests(7).
Regarding biomarkers, atrial natriuretic peptide, especially its pro-B NT fraction, could be a predictor of subsequent dysfunction if elevated during treatment. In asymptomatic patients, it should be interpreted with caution and always in combination with other elements. They are not recommended as the sole follow-up strategy in high-risk patients(12).
Regular exercise is recommended for survivors with normal ventricular function. The American College of Sports Medicine recommends 30-40 minutes of aerobic exercise, five times a week, along with 2 days of higher intensity training. In case of exercise-related symptoms, these should be promptly communicated. Other cardiovascular risk factors (hypertension, diabetes, dyslipidemia and obesity) should be monitored, and tobacco use avoided. During pregnancy, these patients should be closely followed, especially during the first trimester(12).
Finally, chronic fatigue syndrome should be considered, as it has a variable prevalence depending on the series (10-80%), although it is generally considered to affect 30% of children, adolescents and young adults. It consists of a subjective experience of persistent fatigue and severe tiredness, years after diagnosis, unexplained by organic causes, and that does not improve with rest. It is difficult to differentiate from depression. Risk factors include: having received radiotherapy (pulmonary therapy is the most clearly related), psychological stress, relapses, comorbidity of other side effects, female sex, unemployment and affective loneliness. It is inversely correlated with time progression since treatment and with an adequate social and labor integration. There are tools for its diagnosis (PROMIS pediatric fatigue measures, PedsQL MFS). Management is carried out through behavioral therapy (physical exercise, time management, social and occupational integration…), as the efficacy of pharmacological therapies has not been demonstrated(17).
Respiratory sequelae
Pulmonary toxicity is a common late complication, which can have a great impact on the quality of life of survivors, hence the importance in having a high index of suspicion.
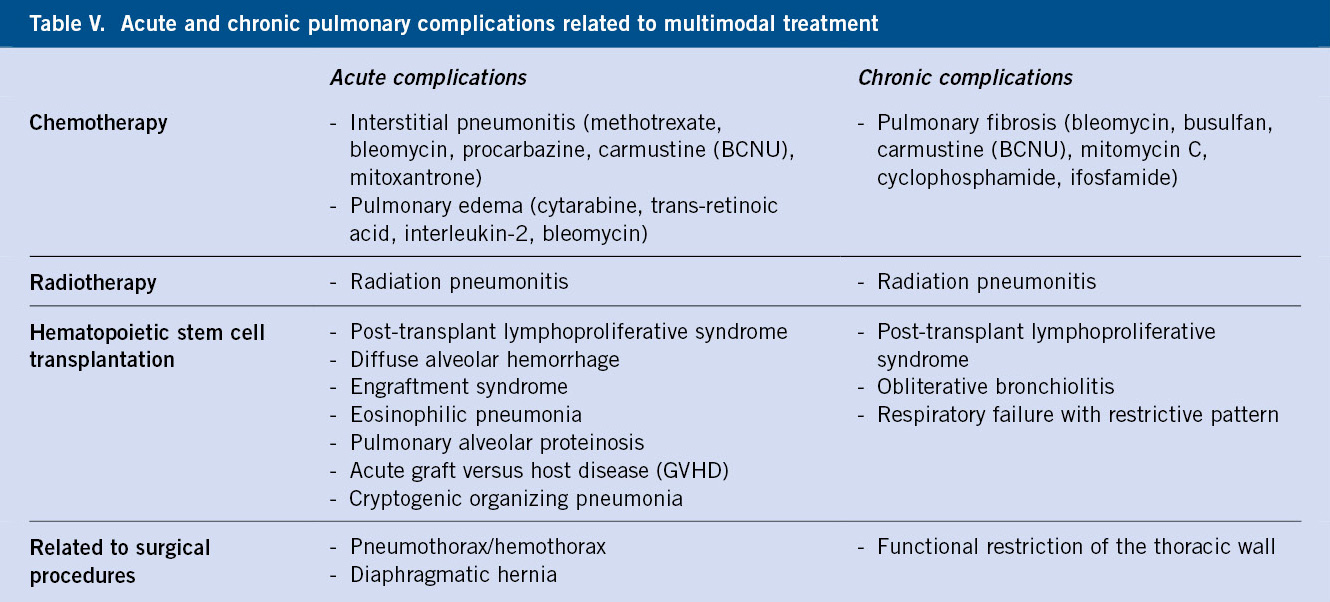
Chronic respiratory tract damage can be related to primary or metastatic disease, infectious complications during treatment, and multimodal therapies themselves. The presentation can be acute or chronic, as summarized in Table V.
The cumulative incidence of pulmonary complications increases with the time elapsed since diagnosis(16), in particular, pulmonary fibrosis and chronic pneumonitis. Radiation therapy can cause oxidative damage to the vascular endothelium and affect the normal development of the chest wall, especially beyond 20 Gy(8,16). In patients receiving a hematopoietic stem cell transplant there is a special vulnerability, due to the conditioning regimens, complications derived from graft versus host disease (GVHD) and respiratory infections associated with the procedure.
During follow-up, watchful vigilance should be kept for guiding symptoms such as persistent dry cough, dyspnea, or exercise intolerance. Lung auscultation should be performed during visits, with baseline O2 saturation and chest X-ray, if symptoms are present. In these cases, the study of lung function is essential, specially assessing restrictive patterns (forced vital capacity, diffusion capacity). Many patients have asymptomatic or oligosymptomatic lung function abnormalities, which should be followed for possible progressive deterioration. The avoidance of tobacco use and its passive inhalation should be advised(11).
Neuropsychological sequelae
Many patients, particularly in high-risk groups, present late neurological, sensory, or cognitive toxicity that impacts their cognitive function and psychosocial integration in adult life.
Neurocognitive sequelae are observed in up to 40-45% of survivors, mainly in those who have suffered a primary or secondary tumor involvement of the central nervous system and in those who have received radiotherapy at that level (CNS tumors, acute lymphoblastic leukemia in previous protocols). Sometimes, there is a functional deficit (visual abnormalities, cranial nerve deficit, hemiparesis, ataxia…) derived from the growth of the tumor itself or from its resection surgery. In the long term, the manifestations can be sensory-motor (aphasia, paresis, focal deficits, peripheral neuropathy…) or neurocognitive (memory, attention, learning, speed of processing and execution of tasks, decreased school performance…). Radiation therapy is especially harmful in children younger than 3-5 years of age. A higher dose is associated with a higher risk of neurocognitive sequelae, in relation to leukoencephalopathy and vascular microangiopathic lesion(11).
At the peripheral nervous system level, sequelae can be observed in patients treated with cisplatin doses > 300 mg/m2 and with vinca alkaloids (especially vincristine, particularly in malnourished children). Cisplatin is often associated with sensory neuropathy. Vincristine rarely causes chronic neuropathy, but if present, it can be multiple (sensorimotor, autonomic, cranial nerves). In these cases, neurophysiological studies are required, as well as offering adequate rehabilitation support, occupational therapy and analgesia if the neuropathy is painful(9).
A follow-up assessment of intellectual, visual, sensory perception, memory, language and learning capacity is recommended, assessing academic performance and behavior in the family and social environment. When stagnation or deficits are observed in any of these areas, early intervention is deemed necessary. Imaging tests (CT angiography, MR angiography) may be required to complete the global assessment, as well as an assessment by Neuropediatrics and Psychology(10).
At a psychological level, there is up to 80% more probability of presenting psychological limitations that affect the quality of life and two times more emotional stress when a comparison with the patient’s siblings is established. Not all tumors present the same pattern of psychological sequelae. Brain tumor survivors display a higher rate of depression, somatization, fatigue, and drowsiness; and those of leukemia, more stress, depression and anxiety during adolescence, compared to other tumors and the general population(8).
Brain irradiation is associated with psychological stress, somatization, a greater feeling of fatigue, poorer physical and mental performance, sleep disruption, and poorer school and social integration. Patients treated with intensive chemotherapy, especially with alkylating agents, present with more stress, anxiety, depression and somatization, especially with alkylating treatments. Risk behaviors associated with psychological dysfunction, high smoking rate and alcohol consumption (in relation to worse socioeconomic status) have been described. In adults, a high rate of suicidal ideation and suicide attempts has been described, with brain tumor survivors having the highest incidence (10.4%)(7). No association has been found with age at diagnosis, time since diagnosis, type of treatment, recurrence or second tumors. Low educational level, low income, unemployment and emotional loneliness have been described as risk factors. In this regard, close monitoring of the social, psychological and emotional situation is relevant.
Sequelae on the senses
Sensory sequelae, especially vision and hearing, can limit the social or work capacity of survivors, so these must be carefully monitored.
The most frequent ocular abnormality is the detection of cataracts. It is related to the use of high and prolonged doses of steroids, radiotherapy at this level (ocular-orbit, cranial, total body, being dose dependent and, especially, if ≥ 2 Gy directly on the lens), busulfan or intrathecal chemotherapy(16). Xerophthalmia and lacrimal duct atrophy can occur and, more rarely: orbital hypoplasia, enophthalmos, keratitis, telangiectasia, retinopathy, maculopathy, optic chiasm neuropathy, papillary damage and glaucoma. The risk increases in case of radiotherapy ≥ 30 Gy on the eye-orbit, in case of treatment with 131-I for thyroid cancer, actinomycin-D or doxorubicin combined with radiotherapy, GVHD, frequent ocular exposure to sunlight and if there is comorbidity (diabetes mellitus, arterial hypertension). Other visual disturbances that may develop are: hypersensitivity to light, blurred vision, diplopia, nyctalopia (night blindness) and alterations in ocular refraction. The risk lasts for more than 20 years beyond the end of treatment(7).
Annual ophthalmological examination is recommended in all survivors, and in those with a history of treatment with corticosteroids or radiotherapy to the eyeball-orbit, orbital tumors, GVHD, treatment with radioactive iodine or abnormal diaphoresis. The use of approved glasses with UV protection is recommended(7).
When it comes to hearing, the most common long-term side effect is ototoxicity. Risk factors include having received high doses of cisplatin or carboplatin, radiation therapy over the head or ear (≥ 30 Gy) and having received therapies such as: aminoglycosides, furosemide, NSAIDs or iron chelators (deferasirox). The usual manifestation is sensorineural deafness, sometimes with tinnitus, and less frequently vertigo, transmission hearing loss and otosclerosis(7). Hearing evaluation is recommended at the end of treatment in patients at risk and then annually for those aged < 6 years (auditory evoked potentials), every 2 years between 6-12 years of age (tonal audiometry of 1,000-8,000 Hz, being best high frequency > 8,000 Hz) and, subsequently, every 5 years in those aged > 12 years. In patients with a ventriculoperitoneal bypass valve, a hearing assessment is recommended at the end of treatment and then every 5 years, even in the absence of other risk factors(7). Avoiding loud noises is recommended.
Smell can be affected in the form of anosmia or chronic rhinitis/sinusitis, usually in patients who have received radiotherapy or surgical resection of a nasal tumor.
Oral and dental sequelae
Patients undergoing chemotherapy before 5 years of age, especially with high-dose cyclophosphamide, are of special risk. One of the main problems of the oral cavity is xerostomia, in general, related to craniofacial radiotherapy, which can improve with frequent intake of liquids, sugar-free candies and artificial saliva tablets. Treatment predisposes to an increased risk of cavities, dental infections, and even speech or sleep disorders. Other dental problems are frequently described (enamel hypoplasia, tooth decay, tooth loss, microdontia, hypodontia, malocclusion). Careful and regular dental hygiene is essential, as well as an assessment by stomatology or dentistry in case of presenting problems. Occasionally, abnormalities in craniofacial development and trismus develop secondary to alterations in the temporomandibular joint(9).
Radiation therapy also increases the risk of developing second neoplasms. Occasionally, the oral cavity may be the site of GVHD involvement in hematopoietic stem cell transplant recipients. An adequate follow-up is important to detect these abnormalities early enough.
Endocrine sequelae
Half of childhood cancer survivors will present at least one late endocrinological disorder in their lifetime, with central nervous system tumors being the most frequently related(7). In the case of craniopharyngioma, abnormality of at least one hormonal axis takes place in 75% of patients. Risk factors are: direct damage from tumor growth itself, that derived from resection or biopsy surgeries, and radiotherapy ≥ 30 Gy. Cranial radiotherapy produces more disturbances in this axis the younger the age of the patient. The most common sequela is short stature due to GH deficiency, particularly with radiation therapy doses > 18 Gy. In adult life, the deficiency of this hormone is associated with metabolic problems such as: increased body fat, altered serum lipid profile, and decreased exercise capacity, bone mineral density and insulin sensitivity. Short stature may, however, be due to other causes (hypothyroidism, precocious puberty, growth impairment due to spinal or long bone radiation, and prolonged corticosteroid therapy)(7). Occasionally, a “catch up” growth occurs reaching the target height with adequate replacement treatment, but it does not always happen(16).
On other occasions, endocrine late effects manifest as central adrenal insufficiency due to ACTH deficiency (very rare, due to direct tumor or surgical damage or radiotherapy dose ≥ 30-50 Gy), central hypothyroidism due to TSH deficiency (cranial RT ≥ 30 Gy), hypogonadotropic hypogonadism due to LHRH, LH or FSH deficiency (second axis affected in order of frequency), prolactin deficiency or central diabetes insipidus due to ADH deficiency (infundibulum involvement by craniopharyngioma, germ cell tumor or optic glioma). The term panhypopituitarism is coined when deficiency of ≥ 3 hormones is present.
Precocious puberty, consisting of early pubertal development, rapid bone maturation, and final height shorter than target, can occur. It is related to radiotherapy dose > 18 Gy, younger age at exposition, and female sex. An annual physical examination is recommended, with anthropometric data and pubertal development monitoring, as well as bone age in case of suspicion. Hyperprolactinemia is observed in some patients, generally related to high-dose irradiation to the pituitary gland or development of second pituitary tumors (adenomas) after treatment. In case of suspicion, serum prolactin will be determined and pituitary MRI performed, as well as referral to Endocrinology(7).
At the thyroid level, hypothyroidism is usually associated with cervical, mediastinal, or spinal radiation therapy. It usually occurs in the first 5 years post-treatment, if exposed to doses > 30 Gy (especially ≥ 45 Gy). Other risk factors are: treatment with therapeutic metaiodobenzylguanidine (MIBG) (despite prophylaxis with oral iodine), anti-GD2 (neuroblastoma), tyrosine kinase inhibitors and secondary to thyroidectomy or ablative treatment with radioactive 131I. Rarely, hyperthyroidism, autoimmune thyroiditis, and thyroid nodules (benign and malignant) may be seen. Hypoparathyroidism secondary to thyroid resection surgery may occur, which requires follow-up and treatment(7).
At the gonadal level, toxicity derived from the use of high-dose alkylating agents (busulfan, melphalan, carmustine, lomustine, cyclophosphamide, ifosfamide, thiotepa, procarbazine, dacarbazine, platinum derivatives) can be observed, especially in combination with radiotherapy on the gonads. Girls are at higher risk if they receive the treatments during or after puberty. Ovarian failure occurs in up to 50% of patients who receive radiation therapy to the gonads (≥ 15 Gy), particularly in association with alkylating agents. Infertility has been described from doses ≥ 5 Gy on the ovaries. Likewise, surgery can produce an iatrogenic menopause (bilateral oophorectomy) or an advance of it (unilateral oophorectomy). In this case, hypergonadotropic hypogonadism would occur, which consequently can lead to an increased risk of premature osteoporosis and cardiovascular problems. Abdominal radiation therapy that involves the uterus increases the risk of intrauterine growth delay and preterm delivery. Annual clinical review of sexual maturation development, menstruation characteristics, hormonal determination (FSH, LH, estrogens) is recommended and, in case of suspected ovarian failure, a bone density scan (DEXA scan) and referral to Endocrinology should be performed(7,11).
In any case, patients whose treatment has placed them at risk for ovarian failure, may have premature menopause with a shortened fertility span, so it is the role of the Primary Care physician to warn of this risk and advise, if the woman is capable and willing to have offspring, to conceive no later than the first years of the fourth decade of life.
In men, germ cells (Sertoli) are sensitive to both chemotherapy and radiotherapy (at doses as low as 2-3 Gy), leading to azoospermia that can be irreversible in the case of radiotherapy or mostly temporary in the case of chemotherapy. Leydig cells are less radiosensitive, but can suffer toxicity from 24 Gy(16). In cases of male hypogonadism due to Leydig cell failure, referral should be made to Endocrinology so as to assess testosterone supplementation in order to avoid the symptoms that its lack generates(9).
In order to anticipate these problems, fertility preservation techniques may be performed in girls (ovarian cortex in prepubertal/postpubertal girls and oocyte vitrification in postpubertal women) and in boys (cryopreservation of semen in postpubertal men and in a few centers, cryopreservation of tissue testicular in prepubertal). The development of consensus guidelines is essential in our setting, since the preservation rate is still low, but efforts are being made to improve this situation(18). Likewise, there are surgical techniques for transposition of the testes or ovaries prior to the administration of scrotal, pelvic or inguinal radiotherapy that would avoid direct irradiation, which is the main risk factor for hypogonadism in these patients.
Regarding follow-up, height (percentile), weight, BMI and pubertal stage should be checked every 6 months until completion of sexual development and final height. Menstrual onset (prepubertal) or re-onset (pubertal), menopausal symptoms (hot flashes, dyspareunia) should be inquired, considering the risk of premature menopause. Referral to Endocrinology should be made if there is failure to thrive, delayed pubertal development, or risk of hypogonadism. It is important to advise on fertility, premature menopause, reproductive advice and individualized risks.
Finally, in the long term, some of the most relevant endocrine and metabolic sequelae are type 2 diabetes mellitus and metabolic syndrome (obesity, arterial hypertension, glucose intolerance – increased insulin resistance and dyslipidemia), which count as main risk factors along with abdominal radiotherapy, total body irradiation, prolonged corticosteroid treatment, inadequate eating habits with excessive caloric intake, sedentary lifestyle and a family history of type 2 diabetes mellitus. Consequently, there is an increase in cardiovascular risk with an increase in morbidity and mortality. Implementation of early dietary measures, increased physical activity, blood pressure and dyslipidemia control are recommended. A tight control of body mass index, blood pressure, lipid profile, blood glucose and Hb A1C will be carried out in clinic(19).
Gastrointestinal sequelae
Gastrointestinal complications can be multiple and heterogeneous depending on the therapy received, the location of the primary tumor and whether there is a history of surgery with organic lesion.
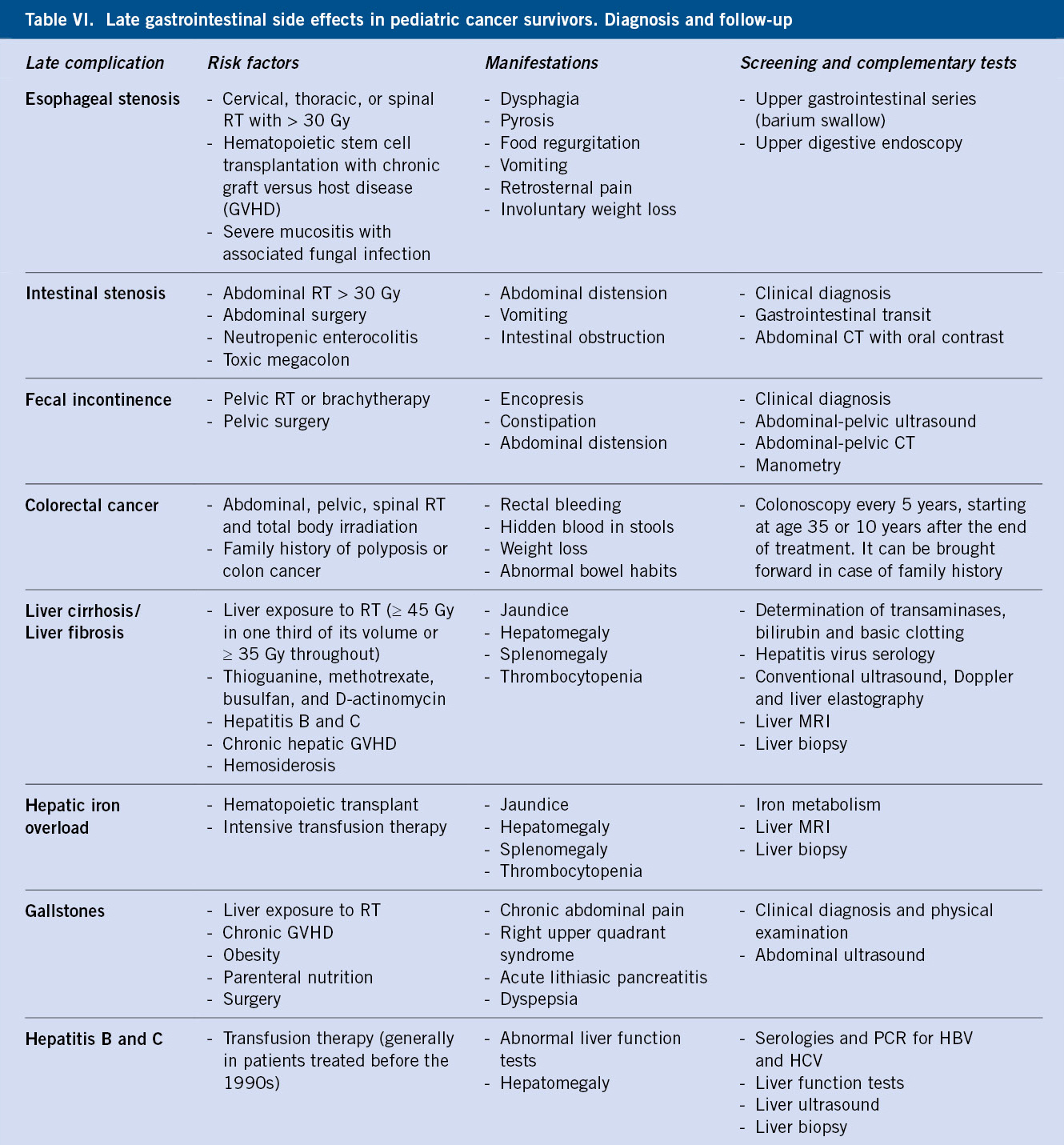
The most relevant gastrointestinal complications are shown in Table VI.
Tumor resection surgery and radiotherapy can have visceral obstructive sequelae. In the latter case, it can cause fibrosis and ischemia due to vascular injury. It can appear as a chronic manifestation in case of intestinal GVHD. Likewise, the specific sequelae derived from surgeries with wide resections should be considered (gastrectomy, pancreaticoduodenectomy, cholecystectomy, splenectomy, intestinal resection with discharge ostomy…). Guiding symptoms such as: dysphagia, heartburn, dyspepsia, reflux, nausea, vomiting, chronic diarrhea, constipation, or progressive weight loss should be monitored. In the cases with a short intestine, hypovitaminosis and lack of absorption of other nutrients ought to be monitored(7).
Chronic hepatotoxicity can appear after a long latency period, being secondary to some chemotherapeutic agents, radiotherapy, obesity, viral hepatitis or iron overload secondary to transfusions. The consumption of hepatotoxic agents, such as alcohol and other drugs of abuse, should be avoided.
Nephrourinary sequelae
Delayed kidney damage in the form of chronic kidney failure is one of the most serious complications in survivors.
The main risk factors for kidney damage at the glomerular and proximal tubular level are abdominal radiation and treatment with high doses of ifosfamide (> 16 g/m2), cisplatin (> 450 mg/m2) and, to a lesser extent, carboplatin. Other factors are the use of nephrotoxic drugs (aminoglycosides, amphotericin B, foscarnet, tacrolimus, and cyclosporine A). It should also be considered that some patients may have a single kidney, due to nephrectomy of their primary tumor (nephroblastoma) or due to metastatic renal involvement(20). Consequently, chronic renal failure, proximal tubulopathy, or Fanconi syndrome may develop(7). Some survivors of Wilms’ tumors are at particular risk, as in addition to having a single kidney, they may have an underlying syndrome (Denys-Drash) and will have received chemotherapy and radiotherapy. High blood pressure is common (30%) during follow-up. In hematopoietic stem cell transplant recipients, nephrotoxicity derived from immunosuppressants, as well as nephritis secondary to BK virus, may occur.
Bladder damage is usually associated with the use of cyclophosphamide or ifosfamide, as well as viral infections (BK virus, adenovirus, cytomegalovirus)(9). There may be lesions of the lower urinary tract, secondary to surgery of solid tumors and radiotherapy/brachytherapy. Secondarily, bladder incontinence (neurogenic bladder), bladder fibrosis, bladder cancer, sexual dysfunction, chronic pelvic pain, bladder floor disorders, fistulas and female vaginal dryness, can also occur(16).
Annual BP control is recommended, with urea, creatinine and electrolytes to assess renal function, venous blood gas, and urine electrolytes. If the post-treatment study is normal, it should be repeated after 5 years. In case of HT, proteinuria or signs of tubulopathy, refer to Nephrology(7).
Musculoskeletal sequelae
Healthy lifestyle habits are important, including regular physical exercise and sufficient amounts of vitamin D and calcium in the diet, as well as the early detection of musculoskeletal problems.
Cranio-spinal radiation, prolonged use of corticosteroids or methotrexate, nutritional alterations and a sedentary lifestyle lead to high risk of this type of sequelae. Soft tissue fibrosis and hypotrophy can occur, as well as a decrease in bone mass with osteopenia/osteoporosis derived from the treatments, prolonged bedridden and radiotherapy on musculoskeletal groups. Other long-term complications are: osteonecrosis (avascular necrosis), exostoses, pathological fractures, bone undergrowth or asymmetric growth, dysmetria and, on some occasions, the sequelae derived from amputations and prosthetic malfunctions(7). With regards to bone mineral density, there is a remarkable margin of recovery throughout adolescence provided a healthy lifestyle, regular physical activity (especially, weight-bearing exercises), controlled sun exposure and optimization of the calcium intake in the diet. Sometimes vitamin D supplements are required. Avoidance of alcohol, tobacco, excessive caffeine, and obesity are recommended(9).
Gynecological sequelae
Breast self-examination and screening based on the individual risk are essential to anticipate breast tumors in adulthood.
In the case of adolescent women, it is important to make the patient aware of the importance of breast self-examination (monthly), as well as a regular gynecological examination. The risk of secondary breast cancer is higher if the patient received chest wall or mediastinal radiation therapy, family predisposition to breast cancer (BRCA1/2 mutations) or history of Li-Fraumeni syndrome (TP53). In these high-risk cases, annual gynecological follow-up is recommended from puberty to age 25, then every 6 months. Annual mammograms should be performed from the age of 25 (other groups recommend it from the age of 30)(10) or from 8 years after treatment (whichever occurs last). Breast MRI is recommended concomitantly with the same timelines as mammograms. In case of mammary hypoplasia, an annual follow-up should take place, referring the patient to Plastic Surgery if breast reconstruction is required, once the pubertal development is completed(7).
Vaccination in the oncohematological patient
Revaccination of the oncohematological patient is of vital importance in the months and years following treatment, in a scheduled manner according to national guidelines and recommendations.
In general, routine vaccinations are suspended during treatment, which added to the fact that some patients have an incomplete vaccination schedule due to their young age at diagnosis, the loss of vaccine immunity acquired by the treatments received and the immunodeficiency maintained by some therapies (rituximab, hematopoietic stem cell transplantation, immunosuppressants), makes them a particularly high infectious risk population at the end of treatment. Given the length of this review, the reader is referred to the periodic updates of the Vaccine Advisory Committee of the AEP (https://vacunasaep.org/documentos/manual/cap-14 y -16, updated as of August 2021).
Risk of premature mortality
There is an increased risk of premature death in survivors, thus, health promotion (Fig. 1) and early diagnosis of complications, as well as second tumors, are of vital importance.
The premature mortality rate of survivors is increased compared to the general population, being higher in the first years of follow-up (cumulative mortality: 6.5% at 10 years, 11.9% at 20 years, and 18.1% at 30 years of diagnosis). The risk is higher in women, brain tumors and Ewing’s sarcoma(7). The main cause is the recurrence of the original disease (67%), which decreases in importance with the passage of time. As the years go by, the toxicity associated with the treatment takes on a more important role in the reduction of life expectancy (late mortality). A 7 times higher risk of dying from cardiovascular events has been described (especially in women, kidney tumors and Hodgkin lymphoma), almost 9 times the risk of dying from lung or respiratory problems (especially patients with AML or neuroblastoma) and more than 2 times from other medical reasons. After 20-30 years of treatment, mortality secondary to second neoplasms is the main cause of death (15 times higher than in the healthy population). Therefore, follow-up strictly restricted to 5 years post-treatment is insufficient in patients who have overcome a tumor in the pediatric age(7-9).
Risk of second neoplasms
Up to 5-20 times higher risk of suffering second tumors histologically different from the initial ones compared to the general population has been described. In general, they are observed at least 2 years after the primary tumor, and within the first 10 years of follow-up, although they can have a latency of up to 30 years(21). The cumulative incidence after 20 years is 3-10%, and after 30 years 5-30%, depending on the series. The primary tumors with the highest incidence of second neoplasms are Hodgkin lymphoma and soft tissue sarcomas. The most common secondary tumors are breast, thyroid, AML, and sarcomas(7). The main risk factors are(6,7,16):
• Chemotherapy. Alkylating agents are associated with myelodysplastic syndromes, which may or may not precede secondary acute myeloblastic leukemias. Their latency is usually 5-7 years and they are accompanied by cytogenetic abnormalities (monosomies and partial deletions of chromosomes 5 and 7). Epipodophyllotoxins (etoposide) are also associated with AML, but this usually develops earlier (2-3 years after completion of treatment). There is a greater risk with doses ≥ 2,000 mg/m2 and associated abnormalities of the MLL gene (which induces 11q23 translocations). Anthracyclines can also cause this type of leukemia, 2-3 years after treatment. The prognosis is unfavorable. Annual full blood count is recommended during long-term follow-up, at least up to 10 years after exposure to chemotherapy. The latency for the development of solid tumors is somewhat longer, on average 14 years. Etoposide increases the risk of secondary Hodgkin lymphoma, neuroblastoma, nephroblastoma, and rhabdomyosarcoma, among others.
• Radiotherapy. It is a relevant risk factor, especially when high doses are received at an early age. It is frequently used in CNS tumors, solid tumors and, historically, in Hodgkin lymphoma and the conditioning with total body irradiation of some transplants. The risk increases as time passes, with a maximum 10-15 years after treatment (although it remains until 30 years later). The types mainly observed are: breast tumors, meningiomas (cranial), bone and soft tissue tumors, thyroid cancer, non-melanoma skin cancer and bladder cancer.
• Hematopoietic stem cell transplantation. It can increase the risk of second neoplasms by up to 8 times compared to the general population (up to 60 times in recipients below the age of 10 years). Its origin is multifactorial (chemotherapy, conditioning radiotherapy and GVHD). Immunosuppressants are associated with the development of post-transplant lymphoproliferative syndrome.
• Cancer predisposition syndromes (Li-Fraumeni, DICER1, NF1-2, telomeropathies…). In recent years, hand in hand with advances in genetics, more are becoming diagnosed. This has allowed the identification of alterations in the germ line or mosaicisms that predispose to one or more tumors, increasing their importance in pediatric Hematology-Oncology. Given the length of the article, we refer the reader to review the bibliography in case of interest(22).
Sufficient information and adequate education about the personalized risks of each patient, promoting self-observation and reducing, as far as possible, risk factors (tobacco, alcohol, sun exposure) is important. Symptoms or signs that may be related to the primary tumor and that suggest a relapse should also be monitored. In this sense, to make an early diagnosis it is essential to have a high index of suspicion, to recognize the risk groups and the “red flags” (warning signs) in each patient(23,24).
The role of the Primary Care pediatrician
As previously discussed in this review, there is not a sole model for pediatric cancer survivor follow-up, and it is essential to adapt it to the individual characteristics and risks of each case, as well as to the real conditions of our healthcare system. In this sense, we have a Pediatric Primary Care network excellently prepared for the promotion of health (Fig. 1) and accompaniment of the patient during their growth. At the hospital level, progress is being made in the development of specialized units to follow-up this type of patients and in their transition to adult care, so we have a mixed care and follow-up model in the routine practice.
Primary Care professionals are a fundamental pillar of multidisciplinary teams, acting as a first line for the diagnosis of sequelae, relapses and second tumors, as well as promoting healthy lifestyle habits. An adequate transmission of the personal history, personalized risks and follow-up plan by hospital health care providers is necessary, as well as a fluid communication between both areas, which will result in a health benefit to our patients. Similarly, the patient and his family should be integrated in the responsibility of long-term care, avoidance of risk behaviors and perception of alarm signs or symptoms, which will prevent loss of adherence to follow-up or low perception of its importance in the long term.
Bibliography
The asterisks show the interest of the article in the opinion of the authors.
1.** Song A, Fish JD. Caring for survivors of childhood cancer: it takes a village. Current Opinion in Pediatrics. 2018; 30: 864-73.
2. Klassen AF, Anthony SJ, Khan A, Sung L, Klaassen R. Identifying determinants of quality of life of children with cancer and childhood cancer survivors: a systematic review. Support Care Cancer. 2011; 19: 1275-87.
3. Shad A, Myers SN, Hennessy K. Late effects in cancer survivors: “the shared care model”. Curr Oncol Rep. 2012; 14: 182-90.
4.** Bhakta N, Liu Q, Ness KK, Baassiri M, Eissa H, Yeo F, et al. The cumulative burden of surviving childhood cancer: an initial report from the St Jude Lifetime Cohort Study (SJLIFE). The Lancet. 2017;390(10112):2569-82.
5. Essig S, Li Q, Chen Y, Hitzler J, Leisenring W, Greenberg M, et al. Risk of late effects of treatment in children newly diagnosed with standard-risk acute lymphoblastic leukaemia: a report from the Childhood Cancer Survivor Study cohort. The Lancet Oncology. 2014; 15: 841-51.
6.** Armstrong GT, Chen Y, Yasui Y, Leisenring W, Gibson TM, Mertens AC, et al. Reduction in Late Mortality among 5-Year Survivors of Childhood Cancer. N Engl J Med. 2016; 374: 833-42.
7.*** Children’s Oncology Group. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers (Internet). 2018. Disponible en: http://www.survivorshipguidelines.org/pdf/2018/COG_LTFU_Guidelines_v5.pdf.
8.*** Skinner R, Wallace W, Levitt GA. Therapy based long term follow up: practice statement: United Kingdom Children’s Cancer Study Group (Late Effects Group) (Internet). Disponible en: https://www.cclg.org.uk/write/MediaUploads/Member%20area/Treatment%20guidelines/LTFU-full.pdf.
9.*** Scottish Intercollegiate Guidelines Network (SIGN), Scotland, Healhcare Improvement Scotland. Long Term Follow Up of Survivors of Childhood Cancer, A National Clinical Guideline (Internet). Disponible en: https://www.sign.ac.uk/media/1070/sign132.pdf.
10.*** Late Effects Taskforce of the Dutch Childhood Oncology Group (DCOG LATER). Guidelines for follow-up after childhood cancer more than 5 years after diagnosis (Internet). Disponible en: https://www.skion.nl/workspace/uploads/vertaling-richtlijn-LATER-versie-final-okt-2014_2.pdf.
11.*** Grupo de trabajo sobre efectos secundarios a largo plazo y segundos tumores de la Sociedad Española de Hematología y Oncología Pediátricas. “Efectos tardíos en supervivientes al cáncer en la infancia”. Cevagraf. 2012.
12.*** Armenian SH, Hudson MM, Mulder RL, Chen MH, Constine LS, Dwyer M, et al. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. The Lancet Oncology. 2015; 16: e123-36.
13. Chow EJ, Leger KJ, Bhatt NS, Mulrooney DA, Ross CJ, Aggarwal S, et al. Paediatric cardio-oncology: epidemiology, screening, prevention, and treatment. Cardiovascular Research. 2019; 115: 922-34.
14. Bansal N, Amdani S, Lipshultz ER, Lipshultz SE. Chemotherapy-induced cardiotoxicity in children. Expert Opinion on Drug Metabolism & Toxicology. 2017; 13: 817-32.
15. Jin Y, Xu Z, Yan H, He Q, Yang X, Luo P. A Comprehensive Review of Clinical Cardiotoxicity Incidence of FDA-Approved Small-Molecule Kinase Inhibitors. Front Pharmacol. 2020; 11: 891.
16.*** Mendoza Sánchez MC. Seguimiento en Atención Primaria del niño oncológico. Cómo detectar las secuelas tardías. Pediatr Integral. 2016; XX(7): 475-84.
17. Christen S, Roser K, Mulder RL, Ilic A, Lie HC, Loonen JJ, et al. Recommendations for the surveillance of cancer-related fatigue in childhood, adolescent, and young adult cancer survivors: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. J Cancer Surviv. 2020; 14: 923-38.
18.** Garrido-Colino C, Lassaletta A, Vázquez MÁ, Echevarría A, Gutiérrez I, Andión M, et al. Situación de la preservación de fertilidad en pacientes con cáncer en nuestro medio: grado de conocimiento, información e implicación de los profesionales. Anales de Pediatría. 2017; 87: 3-8.
19. Friedman DN, Tonorezos ES, Cohen P. Diabetes and Metabolic Syndrome in Survivors of Childhood Cancer. Horm Res Paediatr. 2019; 91: 118-27.
20. Dekkers IA, Blijdorp K, Cransberg K, Pluijm SM, Pieters R, Neggers SJ, et al. Long-Term Nephrotoxicity in Adult Survivors of Childhood Cancer. CJASN. 2013; 8: 922-9.
21.** Choi DK, Helenowski I, Hijiya N. Secondary malignancies in pediatric cancer survivors: Perspectives and review of the literature: Secondary malignancies in pediatric cancer survivors. Int J Cancer. 2014; 135: 1764-73.
22.** Ripperger T, Bielack SS, Borkhardt A, Brecht IB, Burkhardt B, Calaminus G, et al. Childhood cancer predisposition syndromes-A concise review and recommendations by the Cancer Predisposition Working Group of the Society for Pediatric Oncology and Hematology. Am J Med Genet. 2017; 173: 1017-37.
23.** Huerta Aragonés J. Oncología para el pediatra de Atención Primaria (I): signos y síntomas sugerentes de patología neoplásica. Form Act Pediatr Aten Prim. 2014; 1: 4-15.
24.** Huerta Aragonés J. Oncología para el pediatra de Atención Primaria (II): formas de presentación de las diferentes neoplasias infantiles. Form Act Pediatr Aten Prim. 2014; 7: 67-74.
Recommended bibliography
- Children’s Oncology Group. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers (Internet). 2018. Available at:http://www.survivorshipguidelines.org/pdf/2018/COG_LTFU_Guidelines_v5.pdf.
These guidelines are a mainstay in the follow-up, diagnosis and management of late effects in survivors of childhood cancer, with a dilated experience (almost 20 years). They are currently in their 5th version, being updated approximately every 5 years.
- Scottish Intercollegiate Guidelines Network (SIGN), Scotland, Healthcare Improvement Scotland. Long Term Follow Up of Survivors of Childhood Cancer, A National Clinical Guideline. [Internet]. Available in: https://www.sign.ac.uk/media/1070/sign132.pdf.
High quality consensus guidelines, from the year 2013, organized by body organs and systems.
- Skinner R, Wallace W, Levitt GA. Therapy based long term follow up: practice statement: United Kingdom Children’s Cancer Study Group (Late Effects Group) (Internet). Available at: https://www.cclg.org.uk/write/MediaUploads/Member%20area/Treatment%20guidelines/LTFU-full.pdf.
These consensus guidelines focus on both the long-term side effects in the various body organs and those specific to the chemotherapy or radiotherapy treatment received, providing an interesting point of view.
- Grupo de trabajo sobre efectos secundarios a largo plazo y segundos tumores de la Sociedad Española de Hematología y Oncología Pediátricas. “Efectos tardíos en supervivientes al cáncer en la infancia”. Cevagraf. 2012. (Working group on long-term side effects and second tumors of the Spanish Society of Pediatric Hematology and Oncology. “Late effects in survivors of childhood cancer”. Cevagraf. 2012.)
Interesting publication by the SEHOP Working Group on Side Effects, in the form of a textbook. Excellent description of each topic that surpasses in exposure and understanding that obtained, sometimes, in more schematic guides.
- Armenian SH, Hudson MM, Mulder RL, Chen MH, Constine LS, Dwyer M, et al. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. The Lancet Oncology. 2015; 16: e123-36.
Very good article that summarizes the recommendations for cardiac monitoring and surveillance in patients at risk, with special emphasis on risk groups and long-term problems. It is the result of the cooperative work between the Children’s Oncology Group and several European groups, which make up the International Late Effects of Childhood Cancer Guideline Harmonization Group (IGHG).
- Ripperger T, Bielack SS, Borkhardt A, Brecht IB, Burkhardt B, Calaminus G, et al. Childhood cancer predisposition syndromes-A concise review and recommendations by the Cancer Predisposition Working Group of the Society for Pediatric Oncology and Hematology. Am J Med Genet. 2017; 173: 1017-37.
Review of the most relevant cancer predisposition syndromes, with special interest in the risks of pediatric tumor development and specific follow-up recommendations.
| Clinical case |
|
3-year-old boy attended the emergency room due to progressive onset of severe respiratory difficulty. Physical examination revealed marked hypoventilation of the left hemithorax, with increased work of breathing at all levels and decreased oxygen saturation (92%). In the initial chest X-ray, a large hemithorax mass with massive ipsilateral pleural effusion was observed. A chest CT was performed confirming a large mass in the left hemithorax, with doubtful pleural dependence, as well as a severe pleural effusion. After stabilization of the patient and drainage of the pleural effusion, the diagnostic studies were completed in the following days. Thoracic ultrasound-guided percutaneous biopsy of the mass concluded the histological diagnosis of a primitive neuroectodermal tumor of the chest wall. No pulmonary metastases were identified. The patient received treatment according to the protocol in force at that time (Ewing SEOP-2001, treatment group 3). She received induction chemotherapy with 6 VIDE cycles (vincristine, ifosfamide, doxorubicin and etoposide), after which a resection of the tumor followed with reconstruction of the thoracic wall. The response to chemotherapy showed a poor response (80% necrosis), with free surgical margins. A VAC consolidation cycle (vincristine, D-actinomycin, and cyclophosphamide) was administered and, subsequently, an autologous hematopoietic stem cell transplant conditioned with melphalan and etoposide performed. The cumulative dose of cytostatics is as follows: vincristine: 10.5 mg/m2; ifosfamide: 54 g/m2; doxorubicin: 360 mg/m2; etoposide: 4.5 g/m2; cyclophosphamide: 1.5 g/m2; actinomycin-D: 1.5 mg/m2; and melphalan: 140 mg/m2. Finally, 8 weeks after transplantation, radiotherapy with tomotherapy modality was performed with 48 Gy on the primary tumor and 15 Gy on the ipsilateral lung. She attends her follow-up consultations in a timely manner according to the prevailing recommendations in her treatment protocol, with no relapse being observed after 10 years of follow-up. However, at 9 years of age, a lesser growth of the left rib cage is observed, conditioning a significant scoliosis, which requires intervention. Likewise, at 8 years of follow-up, data of mild dilated cardiomyopathy were observed, verified by echocardiography and by magnetic resonance imaging, with mild systolic ventricular dysfunction, for which treatment with enalapril was started.
|
 Follow-up of childhood cancer in Primary Care. How to detect late effects
Follow-up of childhood cancer in Primary Care. How to detect late effects