|
| Topics on Continuous Training |
B. Gómez Cortés, I. Gangoiti Goikoetxea, J. Benito Fernández
Pediatric Emergency Service. Cruces University Hospital. Barakaldo. Biscay
| Abstract
The emergence of new diagnostic tools, such as multiple panels of polymerase chain reaction techniques that currently provide results in a few hours, has facilitated the early etiological diagnosis of these entities. Until microbiological confirmation, several validated scores are also available, that allow us to identify those patients with pleocytosis and a very low risk of presenting bacterial meningitis and reduce the rate of admission and empirical antibiotic treatment. For secondary prevention, it is necessary to know the criteria for close contact in a case of invasive infection by N. meningitidis or H. influenzae. This enables limiting the use of chemoprophylaxis to those individuals for whom it is truly indicated and reducing the risk of developing a secondary case. |
| Resumen
La incidencia de meningitis bacteriana ha disminuido en las últimas dos décadas gracias al desarrollo y, finalmente, inclusión en el calendario vacunal, de las vacunas frente al S. pneumoniae y el N. meningitidis. Sin embargo, siguen presentando una elevada morbimortalidad, al igual que las encefalitis herpéticas. La aparición de nuevas herramientas diagnósticas, como los paneles múltiples de técnicas de reacción en cadena de la polimerasa que, en la actualidad, proporcionan resultados en pocas horas, ha facilitado el diagnóstico etiológico precoz de estas entidades. Hasta la confirmación microbiológica, disponemos, además, de varios scores validados que permiten identificar aquellos pacientes con pleocitosis y muy bajo riesgo de presentar una meningitis bacteriana y reducir la tasa de ingreso y de tratamiento antibiótico empírico. Con vistas a la prevención secundaria, es necesario conocer los criterios de contacto estrecho ante un caso de infección invasiva por N. meningitidis o H. influenzae. Esto permite limitar el uso de la quimiprofilaxis a aquellos individuos en los que verdaderamente está indicada y reducir en ellos el riesgo de desarrollar un caso secundario. |
Key words: Meningitis; Encephalitis; Neisseria meningitides; Streptococcus pneumoniae; Herpes simplex.
Palabras clave: Meningitis; Encefalitis; Neisseria meningitides; Streptococcus pneumoniae; Herpes simplex.
Pediatr Integral 2023; XXVII (6): 341 – 349
OBJECTIVES
• To recognize the symptoms and signs suggestive of CNS infection.
• To adequately carry out the initial stabilization of patients with clinical suspicion of bacterial meningitis or encephalitis, using the ABCDE system.
• To be aware of the diagnostic criteria for encephalitis and low risk criteria for bacterial meningitis in a patient with pleocytosis.
• To prescribe the appropriate antibiotic or antiviral treatment based on the clinical suspicion and the most probable etiological agents in each case.
• To know, if faced with a case of invasive infection by N. meningitidis or H. influenzae, the criteria for considering a person as a close contact, as well as the secondary chemoprophylaxis guidelines recommended in these cases.
Meningitis and meningoencephalitis
Introduction
Infections of the central nervous system are rare entities, but with high morbidity and mortality in case of bacterial meningitis and herpetic encephalitis.
Infections in the pediatric age are a very frequent reason for consultation, both in Primary Care and in the hospital setting, but those that affect the central nervous system (CNS) are less common. Although most of them are viral in origin and have a good prognosis, the morbidity and mortality of these infections remains high, even in our environment.
CNS infections can manifest as:
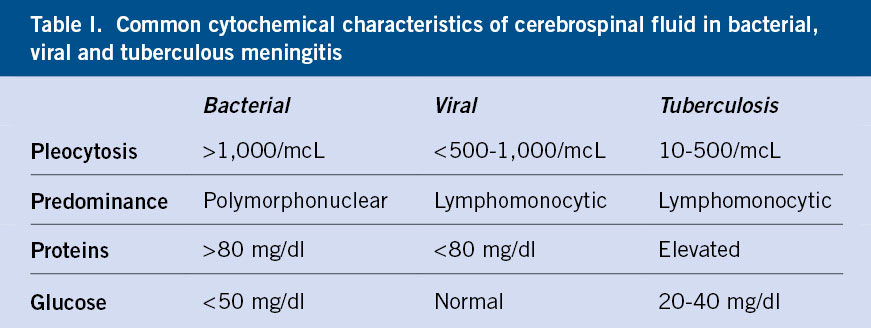
• Meningitis: inflammation of the meninges that affects the pia mater, arachnoid and subarachnoid space. It presents with pleocytosis, which is defined as increased cellularity of the cerebrospinal fluid (CSF): more than 25 cells per microliter in the neonate, more than 10 in the infant between 1 and 2 months and above 5 cells per microliter in patients older than 2 months.
• Encephalitis: inflammation of the brain parenchyma associated with neurological dysfunction, due to infectious or immune-mediated causes. It is a clinical diagnosis in which the guiding symptom is alteration in the mental status, which can present as a decrease or alteration in the state of consciousness, lethargy or personality changes.
• Meningoencephalitis: clinical syndrome characterized by signs and symptoms consisting of inflammation of the meninges and brain parenchyma.
Epidemiology
The vast majority of meningitis are of viral etiology, especially enteroviruses. S. pneumoniae and N. meningitis are the most common causes of bacterial meningitis outside the neonatal period.
Only 5% of meningitis are bacterial. The germs involved vary with age, with group B Streptococcus and E. coli being the most prevalent in the first 2-3 months of life (age period of highest incidence), and S. pneumoniae and N. meningitidis in older infants and children(1). L. monocytogenes, a bacteria classically implicated in neonatal meningitis, is currently a rare cause. Tuberculous meningitis is not common in our environment.
Among the causes of viral meningitis, enteroviruses (85-95%, mainly Echovirus) are the most common. It is difficult to know the exact incidence of CNS viral infections, since the etiological diagnosis is not always made. They are more frequent in the spring, summer and autumn months, and have two peaks of incidence in Pediatrics: under one year of age and between 5-10 years of age.
Regarding meningoencephalitis, members of the Herpesviridae family are the most common cause in our environment(2). Herpes simplex virus type 1 is an important cause of severe sporadic encephalitis that, without antiviral treatment, can progress to coma and death in up to 70%(3). Next in frequency are herpes virus type 2 (which predominates in neonates), Epstein-Barr virus and human herpesvirus 6. Other less common viral causes are: respiratory viruses and rotavirus; in non-immunized patients, measles, rubella and mumps viruses; and in immunocompromised patients, cytomegalovirus. Although rare in our environment, the possibility of emerging viruses should also be taken into account, such as West Nile virus, transmitted by mosquitoes, or the flavivirus causing Central European encephalitis, transmitted by the deer tick (it has also been described through unpasteurized milk).
Pathophysiology
In bacterial meningitis, the symptoms and possible lesions and sequelae are determined by the interrelation of the pathogen’s virulence factors and the host’s immune response and the inflammatory process that this causes.
The process by which bacterial meningitis develops begins with the colonization of the respiratory, gastrointestinal or urinary tract, from where the bacteria invades the bloodstream. Certain host factors (asplenia, complement or antibody deficiency, immunosuppressive treatments…) and having suffered from certain previous viral infections, such as the flu, favor this invasion. Once in the bloodstream, the bacterial polysaccharide capsule inhibits the deposition of adhesins, and phagocytosis and different molecules of the bacterial surface act on specific components of the complement, facilitating the survival of bacteria against the immune system and meningeal invasion. This is facilitated the longer the duration and degree of bacteremia.
In the case of S. pneumoniae and H. influenzae, there is evidence that the choroid plexus is the initial entry point into the ventricular system. Once they reach the CSF, bacteria can multiply rapidly, due to the low concentrations of immunoglobulins and complement that exist there. The accumulation of neutrophils in the CSF is mainly triggered by the complement component C5a and is related to the severity of the disease. Currently, the only adjuvant treatment that has proven useful in reducing mortality in developed countries is dexamethasone, in the treatment of pneumococcal meningitis. However, studies are being developed with other drugs that, in the future, may improve the prognosis of these patients, such as C5a receptor blockers or matrix-metalloproteinase inhibitors (doxycycline)(4).
On the other hand, the bacterial fragments produced after the death of the bacteria due to antibiotic treatment stimulate the immune system, generating a powerful inflammatory response. This has raised the hypothesis that the use of non-bacteriolytic antibiotics, such as daptomycin, can reduce this pro-inflammatory response and prevent neuronal damage, although there are still not enough studies to support it.
As mentioned above, the pathophysiology and clinical translation of bacterial meningitis are defined by the interrelationship of the pathogen’s virulence factors and the host’s immune response. Once the inflammatory process has begun, damage to the endothelium of the blood-brain barrier occurs, causing vasogenic edema, loss of cerebral autoregulation, vasogenic cerebral edema, and increased intracranial pressure. All of them can lead to: hydrocephalus, seizures, motor or sensory deficits, and even coma.
In CNS infections due to enteroviruses, on the contrary, the transmission is usually via the fecal-oral route. The viral particles bind to specific receptors on the enterocytes and cross the intestinal mucosa, reaching Peyer’s patches where they replicate. Transmission through inhalation of infected droplets and subsequent virus replication in the nasopharynx is much less common. In either case, a primary viremia occurs that allows the virus to spread to other organs, such as the liver and spleen, where it replicates again and, subsequently, a secondary viremia. In either of the two periods of viremia, the virus can reach the CNS, although the mechanism by which it does so is still unknown.
Regarding viral encephalitis, HSV-1 is transmitted mainly by direct contact and can reach the CNS by hematogenous
dissemination, direct extension through the cribriform plate or by a neurogenic route. HSV-2 is transmitted mainly through sexual contact, making it a much less common cause of encephalitis, except in the neonate. Viruses can also cause post-infectious encephalitis, triggering an autoimmune response; in this case, the virus would not be detected in microbiological tests performed on CSF.
Clinical manifestations
Signs and symptoms of meningeal inflammation are more frequent and intense in meningitis of bacterial origin. In encephalitis, the guiding symptom is the altered level of consciousness.
• Meningitis: most patients present with fever and symptoms or signs suggestive of meningeal inflammation, although, as in other entities, the symptoms can be more subtle and nonspecific in young infants and especially in neonates. In these two groups, the initial symptoms may be refusal to feed, irritability, vomiting or weakness. In older children and adolescents, the symptoms of meningeal inflammation and intracranial hypertension (ICH) are more evident: headache, photophobia, neck stiffness, and even altered level of consciousness(5). Patients with bacterial meningitis tend to present, more frequently than those with viral meningitis, abnormality of the Pediatric Assessment Triangle, mainly in relation to the side that evaluates appearance(6).
During the examination, certain signs suggestive of meningeal inflammation can be identified:
- Neck stiffness: inability for the chin to touch the chest, presenting a limitation to passive flexion of the neck. Although it is a sign highly suggestive of meningitis, it can appear in other entities, both of neurological (cervical epidural hematoma, subarachnoid hemorrhage, epidural abscess, brain tumor…) and non-neurological origin (cervical lymphadenopathy, retropharyngeal abscess, upper lobe pneumonia, muscle contracture, discitis…).
- Kernig sign: the patient, in a supine position and keeping the hip and knee flexed at 90º, is unable to extend the knee more than 135º or when doing so flexes the opposite knee.
- Brudzinski sign: the supine patient flexes the lower extremities when passive neck flexion is performed.
- Bulging fontanelle: in the infant, it may indicate the presence of ICH; this finding appears to be much less frequent in the neonate(7). It may be absent if dehydration is associated.
As for the neurological findings themselves, the majority of patients with bacterial meningitis present some degree of altered level of consciousness (drowsiness, irritability, lethargy or even coma), this being one of the strongest predictors of mortality or sequelae(8). It is estimated that 20-30% of bacterial meningitis generate seizures, although this percentage is increased in the neonate. In meningitis of viral etiology, both altered level of consciousness and seizures are less frequent signs, while the rest of the symptoms are usually common, although of less intensity.
Certain skin lesions can point to specific etiologies. The presence of petechial or purpuric rash is characteristic of N. meningitidis, although most meningococcal meningitis without associated sepsis do not present it(9). A maculopapular palmoplantar rash, herpangina, or lesions suggestive of hand-foot-mouth disease may be seen in enterovirus meningitis. Enterovirus infections can also present petechial rash, although in this case it is usually punctate elements and the purpuric and hemorrhagic component characteristic of N. meningitidis is exceptional.
• Encephalitis: the defining symptom of encephalitis is altered level of consciousness. This may present as a decreased or fluctuating level of consciousness, lethargy, or changes in personality or behavior. In addition to this, the most common symptoms are fever, seizures and the appearance of focal neurological findings, the most common being cranial nerve palsy, alteration of movements and ataxia(10). Again, neonates and very young infants may initially present with nonspecific symptoms, such as irritability, lethargy, or refusal to feed.
As in meningitis, certain skin lesions can guide the etiology: palmoplantar rash or typical of the mouth-hand-foot in the case of enteroviruses, and vesicular lesions in herpes encephalitis. Other less frequent causes should be considered: history of tick bite (Borrelia or Rickettsia), bites or scratches (Bartonella and Arbovirus) or travel to an endemic area (Rocky Mountain Fever, Rabies, West Nile Virus, Dengue…).
Diagnosis
There are scores that allow identifying patients with pleocytosis and low risk of bacterial meningitis. The clinical picture and certain complementary tests guide the suspicion of encephalitis, but the diagnosis is only given by microbiological confirmation.
The diagnosis of CNS infection is guided by clinical history and physical examination, but the cornerstone for its diagnosis remains to be the study of the CSF. This is the basis of the diagnosis and the interpretation of its different parameters, which usually guides the initial decision making. Other complementary tests, such as blood tests, imaging and neurophysiological tests, can support the clinical suspicion.
• Meningitis: the CSF cytochemistry analysis helps in the initial approach of the etiology of meningitis (Table I).
The acute phase reactants in blood that offer the greatest performance in the identification of bacterial meningitis are:
- Serum procalcitonin (PCT): strongest predictor to differentiate bacterial from viral meningitis. The cut-off point of 0.5 ng/mL has a sensitivity of 99% and a specificity of 83%(11).
- Leukocyte count: Neutrophilia >10,000/mcL is associated with increased risk.
- Serum C-reactive protein, which increases in processes lasting more than 6-12 hours.
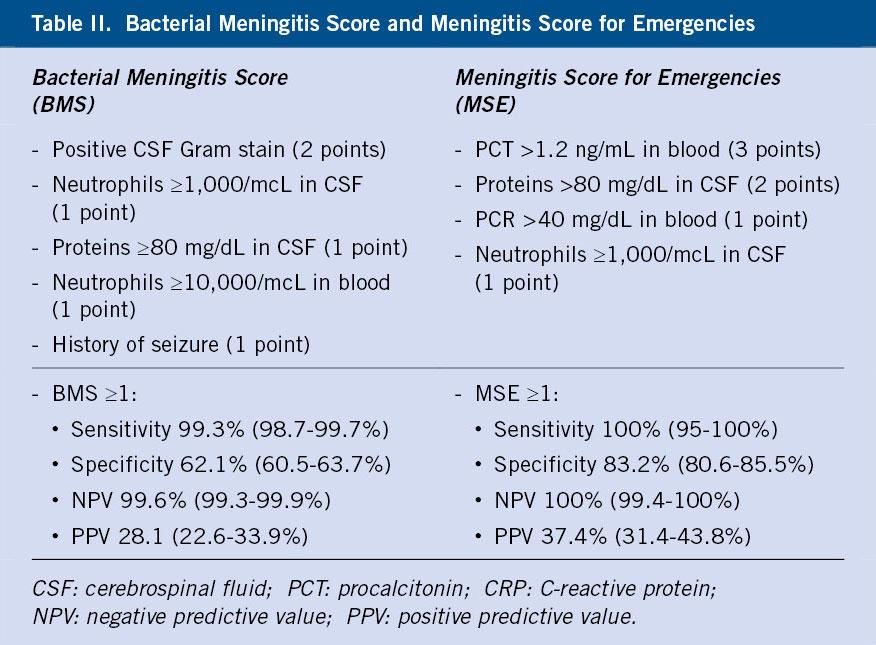
There are validated clinical prediction rules that, by combining clinical and analytical parameters, allow identifying patients with pleocytosis and low risk of bacterial meningitis. Those that have demonstrated greater performance are the Bacterial Meningitis Score (BMS)(12) and, above all, the Meningitis Score for Emergencies (MSE), published more recently(13). Table II shows the parameters included in each of them.
It should be taken into account that they are not applicable to patients who have received antibiotic treatment in the last 72 hours or with any risk factor for bacterial meningitis (poor general condition, purpuric rash, immunosuppressed, CSF shunt or recent neurosurgery). The BMS is validated in patients from 3 months of age and the MSE in patients from one month of age.
• Encephalitis: it is a clinical diagnosis, in which the guiding symptom is the alteration of the mental state, which can present as a decrease or alteration of the state of consciousness, lethargy or personality changes. This major criterion must be accompanied by at least two of the following minor criteria to define possible encephalitis, and three to define probable encephalitis(14). The definitive diagnosis is only given by microbiological isolation.
Minor criteria:
- Fever ≥38ºC in the 72 hours before or after the onset of symptoms.
- Focal or generalized seizures not attributable to previous comorbidity.
- New neurological focality.
- Pleocytosis in CSF.
- Imaging test with alterations of the brain parenchyma suggestive of encephalitis.
- Electroencephalogram with alterations compatible with encephalitis and not attributable to another cause.
The CSF cytochemistry characteristics may vary depending on the causative germ. It is common to find mild pleocytosis (<1,000 mm3), initially with a predominance of polymorphonuclear cells and later of mononuclear cells. Proteins are usually increased in relation to tissue destruction, and glucorrhachia is usually normal. However, there are viral etiologies that may not cause large cytochemical changes, such as parechoviruses.
In both meningitis and encephalitis, microbiological isolation of the causative germ will provide the definitive diagnosis and etiology. Classically, microbiological diagnosis was based on CSF culture. However, cultures take time and sensitivity for diagnosing infections is not optimal. In fact, viral culture has been reviled. In recent years, the emergence of rapid molecular diagnostic techniques, such as the polymerase chain reaction (rt-PCR) technique, based on the amplification of genetic material (DNA or RNA), allow the diagnosis of multiple pathological processes. The new commercialized rt-PCR techniques do not require complex prior manipulations and rapid results are obtained, even at the bedside. Current rapid multiple rt-PCR in CSF allows the presence of genetic material from several microorganisms to be identified in approximately one hour, even in those previously treated with antibiotics. Even so, bacterial culture is still indicated as it also allows the performance of the antibiogram.
In all patients with clinical suspicion of meningitis, bacterial culture and rt-PCR should be requested, at least, for S. pneumoniae, N. meningitidis and enterovirus and parechovirus. If encephalitis is suspected, the study should be expanded with rt-PCR for herpes simplex viruses 1, 2 and 6, and other microorganisms depending on the associated clinical symptoms or the epidemic situation (influenza). It is also advisable to perform an rt-PCR for Mycoplasma pneumoniae in a pharyngeal swab and for enterovirus in stool and/or oropharyngeal sample. In both cases, if available, it is recommended to request a multiple rt-PCR in CSF, which includes all these agents and other less frequent ones.
Differential diagnosis
Although less frequent, a differential diagnosis should be considered with other entities, such as acute disseminated encephalomyelitis or certain autoimmune encephalitis.
In previous sections, the differentiating clinical characteristics between viral and bacterial meningitis have already been discussed. Within the latter, certain pathogens such as M. tuberculosis, T. pallidum and B. burgdorferi (Lyme disease) tend to produce more insidious symptoms.
Other non-infectious processes can also cause inflammation of the CNS and cause symptoms similar to those of viral meningoencephalitis; this is the case of neoplastic diseases, intracranial hemorrhages, exposure to drugs or toxins and autoimmune diseases. Among the latter, acute disseminated encephalomyelitis (ADEM) is considered the leading cause of autoimmune encephalitis in children and adolescents(15). It presents acutely with multifocal neurological deficits, symptoms of encephalopathy and signs of demyelination on brain MRI. Usually, the clinical course is rapidly progressive over several days, and admission to the intensive care unit may be necessary in case of brain stem dysfunction or increased intracranial pressure.
Another diagnosis to take into account is autoimmune encephalitis, due to antibodies against the N-methyl-D-aspartate receptor (anti-NMDA), which is, after ADEM, the second cause of autoimmune encephalitis in the pediatric population(16). In this case, after an initial prodrome of headache and fever that can be confused with any viral process, the patient begins with new symptoms, among which those of the psychiatric sphere stand out (anxiety, agitation, behavioral alterations, hallucinations and even psychotic symptoms). It may be associated with sleep and memory disturbances, seizures, dyskinesia and autonomic instability (hyperthermia, alterations in blood pressure and heart rate…).
Treatment
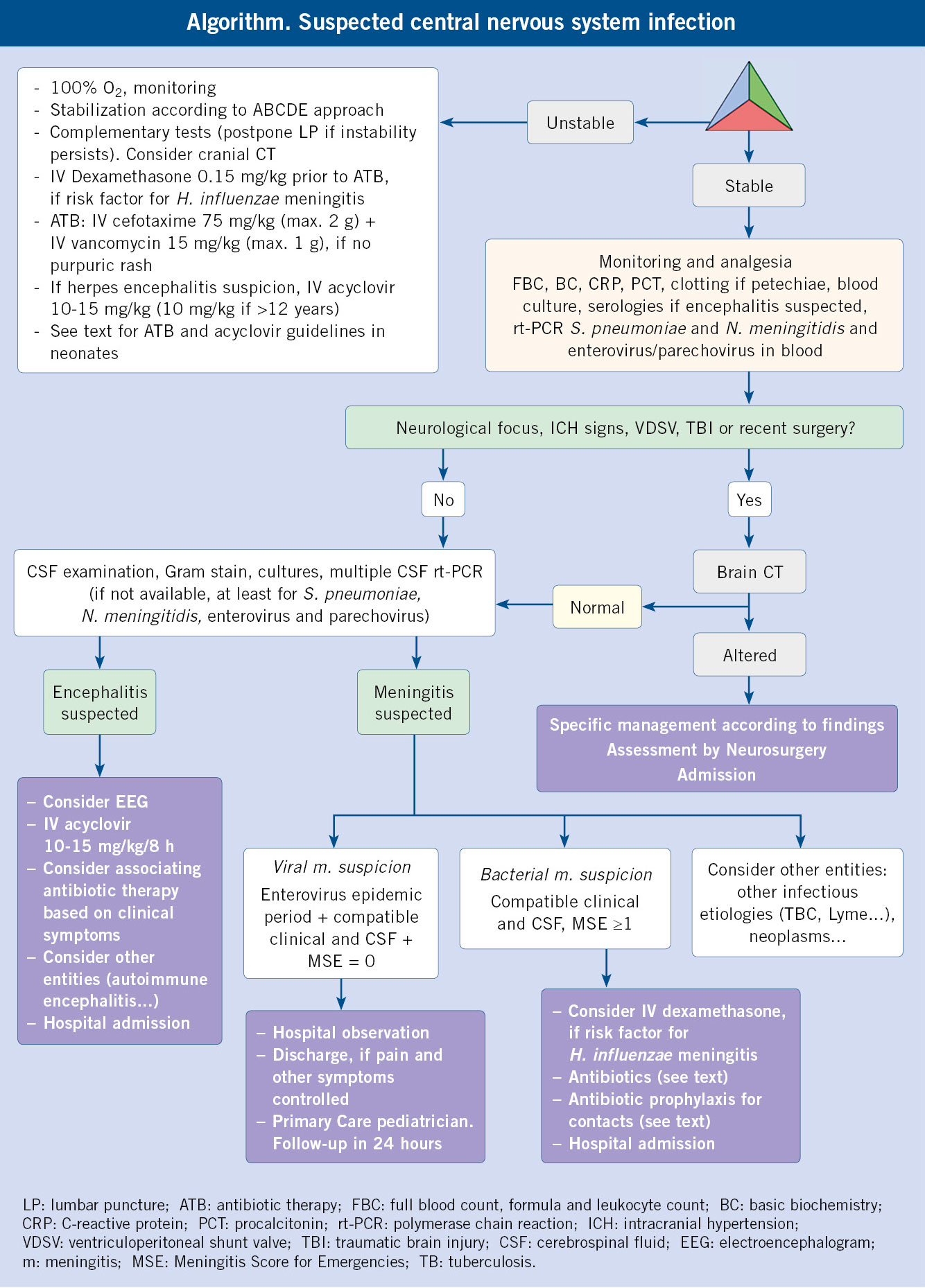
If bacterial meningitis or herpetic encephalitis is suspected, it is essential to carry out adequate initial stabilization using the ABCDE assessment and initiate antibiotic or antiviral treatment early to reduce morbidity and mortality.
The treatment of patients with meningitis or encephalitis is based on stabilization and early initiation of antibiotic or antiviral treatment, in cases with suspected bacterial meningitis or viral encephalitis, respectively.
1. Initial stabilization: it will begin with the assessment of the pediatric evaluation triangle (PET). Patients with bacterial meningitis or encephalitis usually present with CNS dysfunction (less frequently compensated or decompensated shock), although in the initial stages they may present a normal PET.
In every patient with an altered PET, the ABCDE approach and the necessary support measures should be performed according to the identified findings.
• Airway: usually patent, but can be compromised if there is a decrease in the level of conscience.
• Ventilation: determination of respiratory rate and O2 saturation. If appearance is altered and if a non-invasive capnograph is available, determination of exhaled CO2. Administration of oxygen therapy. If polypnea, the possibility of compensatory metabolic acidosis that could indicate an associated sepsis situation should be assessed.
• Circulation: determine heart rate and blood pressure. Evaluate capillary refill, peripheral pulses, coloration and thermal gradient. The alteration of any of these data may indicate an alteration in perfusion and, therefore, an associated shock situation. Peripheral catheterization and, in case of tachycardia, hypotension or signs of poor perfusion, start expansions with isotonic fluids at 10 ml/kg, assessing response and repeating if necessary until these parameters improve. If there is no shock situation, it is recommended to wait until the sodium level is available to start the most appropriate fluid therapy: maintenance fluid therapy, if the sodium level is ≥135 mEq/L; and restriction to 60-75% of the maintenance rate, if sodium levels are <130 mEq/L, due to the risk of developing a syndrome of inappropriate antidiuretic hormone secretion (SIADH).
• Neurological status: assess alertness (AVPU scale), characteristics of the pupils that may indicate signs of ICH, and the presence of anomalous postures or movements. If the child only responds to painful stimuli, consider Glasgow <9 and assess the need to instrument the airway. If an active seizure occurs, give priority to maintaining the airway and, in the event of a seizure lasting more than 5 minutes, administer a benzodiazepine (intravenous as the first option, intramuscular, intranasal or buccal as an alternative, if venous access is not available). Determine blood glucose and assess pain, administering appropriate analgesia if necessary.
• Exposure: identify skin lesions that can guide the etiology of the condition and determine the temperature. Protect from hypothermia.
2. Antibiotic or antiviral treatment:
• Antibiotic treatment: in previously healthy patients with normal PET and suspected meningitis, the decision to start antibiotic treatment will be determined by the presence or absence of clinical risk factors and the BMS or MSE score. In those patients without risk factors and with scores indicating a very low risk (BMS/MSE = 0), supportive treatment and clinical control are appropriate.
In patients with altered PET, risk factors or BMS/MSE scores ³1, antibiotic treatment will be initiated. Ideally, it is recommended to administer the first dose once the lumbar puncture has been performed, so as not to affect the performance of the Gram stain and CSF culture. However, in the case of a patient with a high suspicion of unstable bacterial etiology or in whom the lumbar puncture must be delayed, antibiotic therapy will be initiated once the blood culture has been extracted, to avoid delays affecting the prognosis.
Due to the limitation to cross the hematoencephalic barrier, the concentration that most antibiotics reach in the CSF is only 10-20% of that reached in the blood. However, this increases in the first 24 hours of the infectious process, due to the greater permeability caused by the inflammatory response. Empirical treatment in previously healthy patients, outside the neonatal period, is:
- Vancomycin 15 mg/kg/6 h (max. 1 g/6 h).
- Ceftriaxone 50 mg/kg/12 h (plus 2 g/12 h) or cefotaxime 75 mg/kg/6 h (max. 2 g/6 h).
In areas with low prevalence of cephalosporin resistant S. pneumoniae (including intermediate resistance), monotherapy with ceftriaxone/cefotaxime may be considered.
In neonatal meningitis, empirical treatment will be:
- Ampicillin 75 mg/kg/6 h in >7 days and 100 mg/kg/8 h in ≤7 days.
- Ceftazidime 50 mg/kg/8 h.
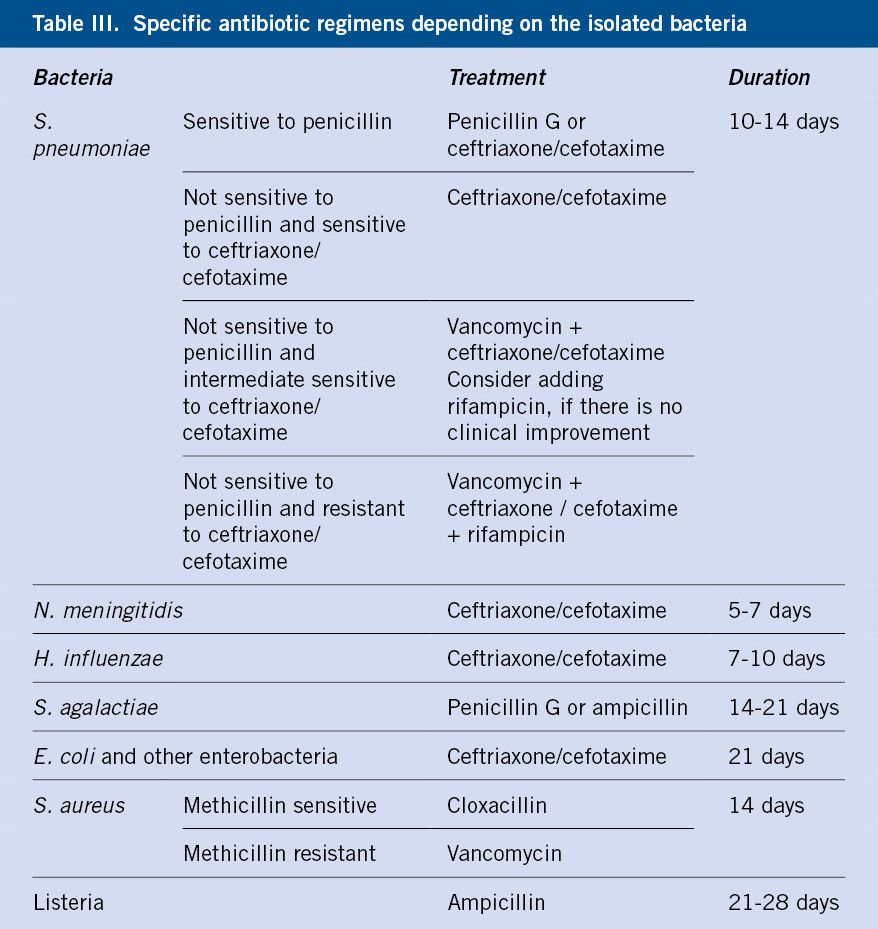
Once the causative bacteria is isolated, the antibiotic treatment will be modified to the specific regimen (Table III)(17). Repeating the lumbar puncture will be considered if there is no clinical improvement within 48 hours (fever may persist longer), there is clinical worsening after initial improvement, and in meningitis due to gram-negative bacilli.
• Antiviral treatment: most patients with viral meningitis do not require antiviral treatment. Empirical treatment will be started with acyclovir in patients with clinical suspicion of encephalitis. Dose:
- Neonate: 20 mg/kg/8 h.
- <12 years: 10-15 mg/kg/8 h.
- ≥12 years: 10 mg/kg/8 h.
• Dexamethasone: different studies have analyzed the usefulness of dexamethasone as an adjuvant treatment in patients with bacterial meningitis. Several meta-analyses show that it reduces the rate of deafness in Haemophilus meningitis, but without differences in meningitis due to other bacteria. Nor does it reduce mortality or the rate of other sequelae(18). The benefit appears to be greater when administered in short-term processes and it should be taken into account that its use could reduce the penetration of vancomycin through the blood-brain barrier. Given that in our environment, Haemophilus meningitis is currently very rare, its use only seems indicated in patients with risk factors for it (sickle cell anemia, asplenia…). If administered, It should be done before or at the same time as the first dose of antibiotic, using a regimen of 0.15 mg/kg/6 h for 48 hours. Adjuvant treatment with dexamethasone is also indicated in tuberculous meningitis. In this case, the treatment is continued for two months.
Prognosis: mortality from bacterial meningitis is estimated at 4-5% in developed countries and double that in developing countries. Among the main neurological sequelae that patients can develop, deafness and the development of secondary epilepsy stand out. Pneumococcal meningitis presents both higher mortality (7-15%) and risk of deafness. Hypoglycorrhachia <20 mg/dl is also related to a higher risk of deafness, while a Glasgow scale <9 at the initial assessment and the persistence of seizures beyond 72 hours after starting antibiotic treatment are associated with a higher risk of neurological sequelae.
The development of medium and long-term sequelae in patients with enterovirus or parechovirus meningitis, outside the neonatal period, is exceptional, although they can persist for several weeks with asthenia, irritability and difficulties concentrating. Some studies suggest a possible impact on cognitive development, but the evidence to date is low. On the contrary, the development of neurological sequelae in herpes encephalitis is high, with the prevalence in some studies being up to 50-60%. These include epileptic disorders, learning difficulties, developmental delays and behavioral disorders(19). The prevalence is higher in neonatal encephalitis, in which a mortality rate of around 4% is also described.
Prevention
Vaccination against H. influenza, S. pneumoniae and N. meningitidis has reduced the incidence of bacterial meningitis and its complications. The criteria for chemoprophylaxis in contacts of cases of invasive infection must be known.
1. Primary prophylaxis: the current vaccination schedule, proposed by the Interterritorial Council of the Spanish National Health System, includes the vaccines against H. influenzae B, S. pneumoniae (13-valent conjugate vaccine) and N. meningitidis. Additionally, administration of the 23-polysaccharide S. pneumoniae vaccine is recommended for immunosuppressed patients with asplenia, complement deficiency, advanced chronic kidney disease, chronic cardiovascular, respiratory or liver diseases, CSF fistula, or cochlear implant.
2. Secondary prophylaxis: it is recommended to prescribe rifampicin in the following cases, see doses in table IV.
a. N. meningitidis(20):
• Close contacts with the index case:
- All people living at the address of the index case.
- All people who have spent the night in the same room as the case, any of the 10 days prior to their hospitalization.
- Other people who have had direct contact with the patient’s nasopharyngeal secretions in the 10 days preceding their hospitalization, such as intimate kisses on the mouth.
- Health personnel who have performed resuscitation maneuvers, mouth-to-mouth or maskless endotracheal intubation.
• In daycare centers and early childhood education centers: all children and classroom staff. If children from several classrooms in the same center have activities in common, all of them will be considered as contacts. This fact will be evaluated with special attention in daycare centers; since, generally, the separation of children in classrooms is less rigid than in schools. School bus buddies, recesses or other time-limited activities will not be considered contacts.
• It is not indicated to administer chemoprophylaxis to students who attend the same class or primary, secondary and university education center, unless they have had close contact.
b. H. influenzae type B:
• Cohabitants and people who have contacted the index case four or more hours a day, at least five of the seven days prior to hospitalization, if they are in contact with children under 4 years of age who have not received complete vaccination against H. influenzae type B or with immunosuppressed individuals.
• Contacts in daycare, when there are 2 cases of invasive disease in less than 60 days.
Role of the Primary Care pediatrician
• To recognize the symptoms and signs suggestive of infection of the CNS so as to early initiate adequate management of these patients.
• To adequately carry out the initial stabilization of patients with clinical suspicion of bacterial meningitis or encephalitis, through the ABCDE system, carrying out measures that reduce morbidity and mortality.
• To activate the Emergency system appropriately, with the aim of transferring these patients to a hospital setting.
• To know the indications for secondary chemoprophylaxis in contacts of cases of invasive infection due to N. meningitidis or H. influenzae.
• To promote vaccination of boys and girls to reduce the incidence of these infections.
Conflict of interests
There is no conflict of interest in the preparation of the manuscript. Declaration of interests: none.
Bibliography
The asterisks show the interest of the article in the authors’ opinion.
1.* Ouchenir L, Renaud C, Khan S, Bitnun A, Boisvert AA, McDonald J, et al. The Epidemiology, Management, and Outcomes of Bacterial Meningitis in Infants. Pediatrics. 2017; 140: e20170476.
2.** Granerod J, Ambrose HE, Davies NW, Clewley JP, Walsh AL, Morgan D, et al; UK Health Protection Agency (HPA) Aetiology of Encephalitis Study Group. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis. 2010; 10: 835-44.
3.** Rozenberg F. Herpes simplex virus and central nervous system infections: encephalitis, meningitis, myelitis. Virology (Montrouge). 2020; 24: 283-94.
4.* Bewersdorf JP, Grandgirard D, Koedel U, Leib SL. Novel and preclinical treatment strategies in pneumococcal meningitis. Curr Opinion Infect Dis. 2018; 31: 85-92.
5. Curtis S, Stobart K, Vandermeer B, Simel DL, Klassen T. Clinical features suggestive of meningitis in children: a systematic review of prospective data. Pediatrics. 2010; 126: 952-60.
6.* Johansson Kostenniemi U, Norman D, Borgström M, Silfverdal SA. The clinical presentation of acute bacterial meningitis varies with age, sex and duration of illness. Paediatr Act. 2015; 104: 1117-24.
7.** Martínez E, Mintegi S, Vilar B, Martínez MJ, López A, Catediano E, et al. Prevalence and predictors of bacterial meningitis in young infants with fever without a source. Pediatr Infect Dis J. 2015; 34: 494-8.
8.** Roine I, Peltola H, Fernández J, Zavala I, González Mata A, González Ayala S, et al. Influence of admission findings on death and neurological outcome from childhood bacterial meningitis. Clin Infect Dis. 2008; 46: 1248-52.
9.** Gangoiti I, Valle JR, Sota M, Martínez-Indart L, Benito J, Mintegi S, et al. Characteristics of children with microbiologically confirmed invasive bacterial infections in the emergency department. Eur J Emerg Med. 2018; 25: 274-80.
10.*** Britton PN, Dale RC, Blyth CC, Clark JE, CraFord N, Marshall H, et al. Causes and Clinical Features of Childhood Encephalitis: A Multicenter, Prospective Cohort Study. Clin Infect Dis. 2020; 70: 2517-26.
11.** García S, Echevarri J, Arana-Arri E, Sota M, Benito J, Mintegi S, et al. Outpatient management of children at low risk for bacterial meningitis. Emerg Med J. 2018; 35: 361-6.
12.*** Nigrovic LE, Malley R, Kuppermann N. Meta-analysis of bacterial meningitis score validation studies. Arch Dis Child. 2012; 97: 799-805.
13.*** Mintegi S, García S, Martín MJ, Durán I, Arana-Arri E, Fernández CL, et al; Meningitis Group of the Spanish Society of Pediatric Emergencies. Clinical Prediction Rule for Distinguishing Bacterial from Aseptic Meningitis. Pediatrics. 2020; 146: e20201126.
14.** Venkatesan A, Tunkel AR, Bloch KC, Lauring AS, Sejvar J, Bitnun A, et al. Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortium. Clin Infect Dis. 2013; 57: 1114-8.
15.* Cole J, Evans E,Mwangi M, Mar S. Acute disseminated encephalomyelitis in children: an updated review based on current diagnostic criteria. Pediatr Neurol. 2019; 100: 26-34.
16.* Dalmau J, Armangué T, Planagumà J, Radosevic M, Mannara F, Leypoldt F, et al. An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: mechanisms and models. Lancet Neurol. 2019; 18: 1045-57.
17.* Garrido Colino C. Bacterial meningitis (v.1.1/2008). Guía_ABE. Infecciones en Pediatría. Guía rápida para la selección del tratamiento antimicrobiano empírico. (ABE_Guide. Infections in Pediatrics. Quick guide for the selection of empiric antimicrobial treatment). 2008. Available in: http://www.guia-abe.es.
18.** Brouwer MC, McIntyre P, Prasad K, van de Beek D. Corticosteroids for acute bacterial meningitis. Cochrane Database Syst Rev. 2015; 2015: CD004405.
19.* Rismanchi N, Gold JJ, Sattar S, Glaser C, Sheriff H, Proudfoot J, et al. Neurological Outcomes After Presumed Childhood Encephalitis. Pediatr Neurol. 2015; 53: 200-6.
20.*** Departamento de salud. Gobierno vasco. Protocolos de vigilancia Epidemiológica. Enfermedad meningocócica invasiva. (Health Department. Basque government. Epidemiological surveillance protocols. Invasive meningococcal disease). 2019. Available in: https://www.euskadi.eus/contenidos/informacion/vigilancia_protocolos/es_def/adjuntos/Protocolo-EMI_19062019_cast.pdf.
21. Justo Ranera A, Soler-Palacín P, Codina MG, Gonzalo de Liria CR. Meningoencefalitis viral. (Viral meningoencephalitis). Pediatr Integral. 2018; XXII: 282-93. Available in: https://www.pediatriaintegral.es/publicacion-2018-09/meningoencefalitis-viral/.
Recommended bibliography
- Britton PN, Dale RC, Blyth CC, Clark JE, Crawford N, Marshall H, et al. Causes and Clinical Featurees of Childhood Encephalitis: A Multicenter, Prospective Cohort Study. Clin Infect Dis. 2020; 70: 2517-26.
Multicenter study that includes more than 500 cases of patients with suspected encephalitis, providing information on etiology, clinical characteristics and progress. A third of the cases were associated with mortality or short-term sequelae.
- Nigrovic LE, Malley R, Kuppermann N. Meta-analysis of bacterial meningitis score validation studies. Arch Dis Child. 2012; 97: 799-805.
Meta-analysis of 8 studies and more than 5,000 patients, which analyzes the performance of the BMS to identify patients with pleocytosis and low risk of meningitis, obtaining a sensitivity of 99.3% and a specificity of 62.1%.
– Mintegi S, García S, Martín MJ, Durán I, Arana-Arri E, Fernández CL, et al; Meningitis Group of the Spanish Society of Pediatric Emergencies. Clinical Prediction Rule for Distinguishing Bacterial from Aseptic Meningitis. Pediatrics. 2020; 146: e20201126.
Derivation and validation study of the Meningitis Score for Emergencies, which includes more than 1,000 patients treated in 25 Spanish hospitals. It obtained a sensitivity of 100% and a specificity of 83.2% to identify patients at low risk of bacterial meningitis.
– Departamento de salud. Gobierno vasco. Protocolos de vigilancia Epidemiológica. Enfermedad meningocócica invasiva. (Health Department. Basque government. Epidemiological surveillance protocols. Invasive meningococcal disease). 2019. Available in: https://www.euskadi.eus/contenidos/informacion/vigilancia_protocolos/es_def/adjuntos/Protocolo-EMI_19062019_cast.pdf.
Document prepared by the health department of the Basque Government with information on incidence, case and contact definitions of invasive meningococcal infection and recommendations for secondary chemoprophylaxis.
| Clinical case |
|
A 5-year-old girl, with no relevant medical or surgical history, was transferred in an advanced life support mobile unit, monitored and receiving 100% oxygen therapy with a reservoir mask. She has presented an episode of lack of consciousness lasting 10 minutes, without response to verbal stimuli and without associating limb movements. Since it subsided, she has presented generalized hypotonia and reports that she feels sleepy. Upon the arrival of advanced life support team, she responds to verbal stimuli. Her family reported a mild cold that had been going on for several days, with fever in the last 24 hours, in which they noticed she was less active than usual and sleepier. She was transferred without incidents. Upon arrival at the hospital, about two hours after the described episode, she was afebrile, with normal vital signs for her age, including blood glucose and capnography. She vomits twice and reports frontal headache. In the neurological examination, it is notable that, despite reacting to verbal stimuli, she has difficulty fixating her gaze, bradylalia, and bradypsychia. The rest of the examination is unremarkable, except for increased osteotendinous reflexes in the lower extremities. The gait is normal, there is no dysmetria and there are no positive meningeal signs. The analysis shows no electrolyte alterations, CRP and PCT values are negative, presenting only a slight leukocytosis with neutrophilia (13,500 leukocytes/mcL with 75% neutrophils). The study is completed with: determination of toxic substances in urine (negative), brain CT (no alterations) and lumbar puncture (clear-appearing fluid, without pleocytosis and with normal glucorrhachia and proteinorrachia).
|
 Meningitis and meningoencephalitis
Meningitis and meningoencephalitis