|
| Topics on Continuous Training |
M. González-Valcárcel Espinosa1, L. Otero Durán2, S.M. Caballero Martín3, P. Malillos González4, A.B. Escobar Izquierdo1, M.C. López Menau1
1Consultant in Pediatrics. Neonatology Unit. Toledo University Hospital Complex. 2Consultant in Pediatrics. Neonatology Unit. Ourense University Hospital Complex. 3Consultant in Pediatrics. Neonatology Unit. Gregorio Marañón General University Hospital. Madrid. 4Primary Care Pediatrician. La Ventilla Health Center. Madrid
| Abstract
Within the field of Neonatology, prematurity management has undergone significant advances in recent decades, improving this patient group’s viability, morbidity, and mortality outcomes. Respiratory pathology and hemodynamic management are two important pillars in the approach to these newborns. Neurodevelopmental centered care represents a field for improvement in neurobehavioral evolution, with the support of physical examination and transcranial ultrasound monitoring. This chapter aims to develop the outlines of diagnosis and treatment for existing diseases in this patient group. |
| Resumen
Dentro del mundo de la Neonatología, el manejo de la prematuridad ha sufrido importantes avances en las últimas décadas, mejorando ampliamente los resultados de viabilidad y morbimortalidad en este grupo de pacientes. La patología respiratoria y el manejo hemodinámico son dos importantes pilares en el manejo de estos recién nacidos. Los cuidados centrados en el neurodesarrollo suponen un campo de mejora en la evolución de la neuroconducta, apoyándonos en la exploración y el seguimiento ecográfico transcraneal. Procuraremos desarrollar en este capítulo las pinceladas de diagnóstico y tratamiento por patologías existentes en este grupo de pacientes. |
Key words: Respiratory distress; Neurodevelopment; Bronchopulmonary dysplasia; Patent ductus arteriosus; Necrotizing enterocolitis
Palabras clave: Distrés respiratorio; Neurodesarrollo; Displasia broncopulmonar; Ductus arterioso persistente; Enterocolitis necrotizante.
Pediatr Integral 2024; XXVIII (3): 160 – 170
OBJECTIVES
• To familiarize with the classification of prematurity according to gestational age and birth weight.
• Preterm respiratory distress. To understand the progression of respiratory management in this group of patients, using thoracic ultrasound at the incubator level as a support tool in respiratory management.
• To know the management of ductus arteriosus in prematurity and new support tools such as functional echocardiography, aiding in the decision-making process when treating it.
• Necrotizing enterocolitis. The role of breast milk as essential nutrition for the preterm and prevention of digestive pathology. To recognize the value of breast milk banks and personalized nutrition units.
• Neurological pathology in prematurity: intraventricular hemorrhage and periventricular leukomalacia. Care focused on neurodevelopment and family integration in the daily care of these children. Multidisciplinary management with routine examinations, observation of the important role of general movements and evolving transcranial ultrasound control during admission.
• Description, management and prognosis of retinopathy of prematurity.
 |
 |
Prevalent pathologies in prematurity
Introduction
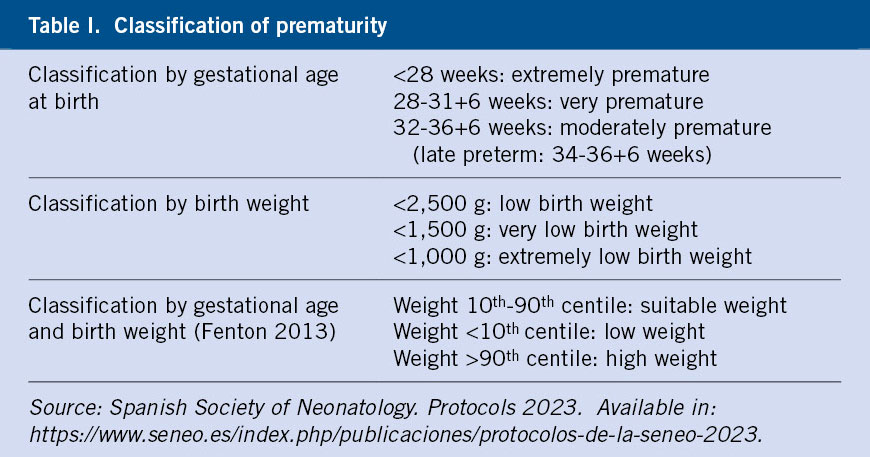
Prematurity includes newborns born before 37 weeks of gestational age (GA) and constitutes 5-18% of total births worldwide and 6.3-7.4% nationally. This group of patients will be classified according to GA and birth weight(1) (Table I).
The risk of morbidity and mortality is directly related to the weeks of gestation at birth and weight, with birth in specialized centers by level of care according to the GA playing an essential role. In this chapter we will address the management of pathologies in preterm newborns (PTNB).
Need for resuscitation in the delivery room(1,2)
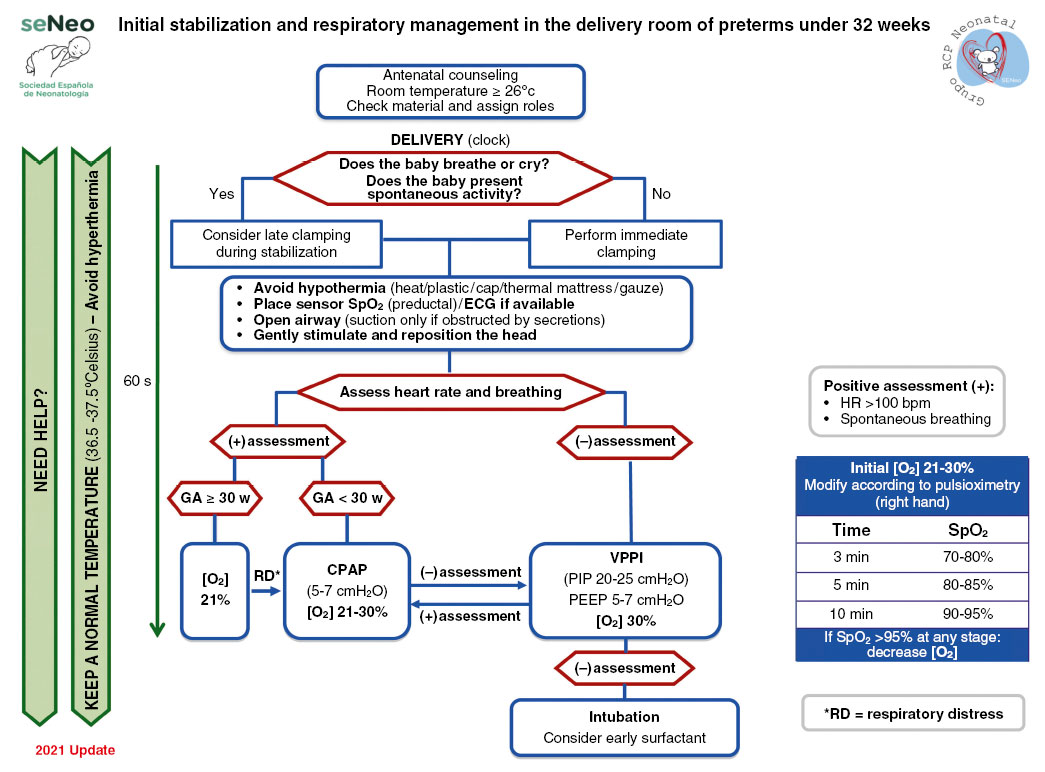
The need for resuscitation and support at birth is greater in the group of premature newborns (Fig. 1).
Figure 1. Initial stabilization and respiratory management in the delivery room of premature babies under 32 weeks. Source: Spanish guide for neonatal stabilization and resuscitation 2021: Analysis, adaptation and consensus on international recommendations. Available in: https://www.analesdepediatria.org/en-spanish-guide-for-neonatal-stabilization-articulo-S2341287922000138. CPAP: continuous positive airway pressure; GA: gestational age in weeks; HR: heart rate; IPPV: intermittent positive pressure ventilation; PEEP: Positive end-expiratory pressure; PIP: peak inspiratory pressure; SpO2: oxygen saturation.
The group of the Spanish Society of Neonatology(1) sets certain limits of viability, with children under 23 weeks of GA not being candidates for resuscitation, providing them with comfort measures at birth. Those older than 24 weeks GA will be candidates for active resuscitation. The group of newborns from 23 to 25 weeks GA constitute a gray area in which the opinion of the parents will be taken into account.
Preterm respiratory distress(1,3,4)
Definition. Etiopathogenesis
It constitutes one of the most prevalent pathologies in PTNBs, causing an interruption in lung development and a surfactant deficiency, with the consequent respiratory difficulty immediately after birth.
Pathophysiology
Surfactant deficiency leads to a loss of surfactant function, making these lungs more rigid and prone to alveolar collapse, in addition to the premature infant’s reduced muscle strength.
Clinical characteristics
Nasal flaring, polypnea, expiratory moaning (as a mechanism for generating autoPEEP – positive end-expiratory pressure–) and sub, inter and suprasternal indrawing. The Silverman scale classifies clinical severity.
Diagnosis(1,3,4)
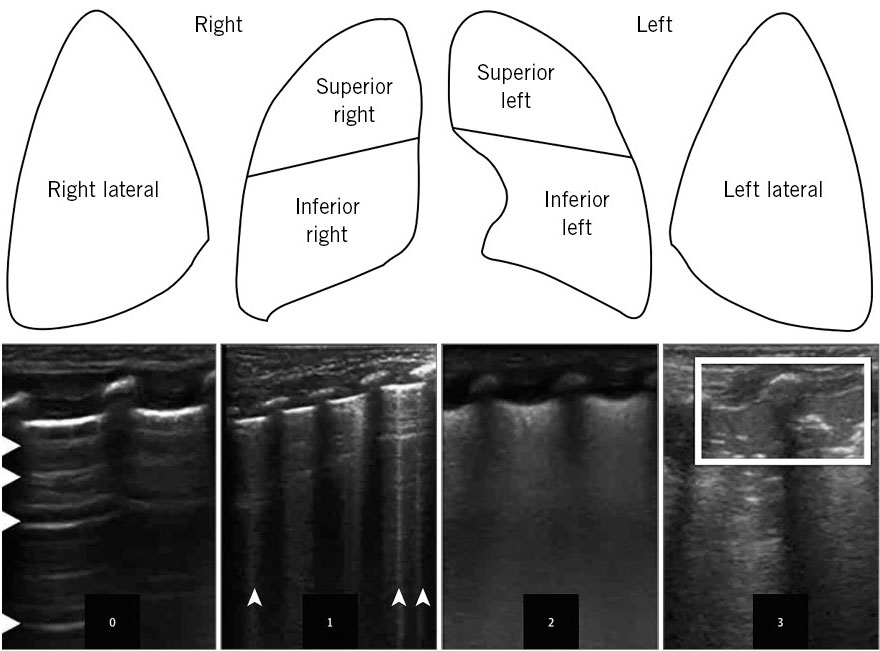
The need to maintain a preductal oxygen saturation of 90-94% with a FiO2 requirement (fraction of inspired oxygen) greater than 30% and alteration in ventilation. Thoracic ultrasound can be useful in early diagnosis and allows the severity to be graded using the lung ultrasound-score (LUS-score)(4) (Fig. 2).
Figure 2. Assessment scale and LUS-score (lung ultrasound-score) in thoracic ultrasound of immediate respiratory distress of preterm. 0: normal aeration pattern-A; 1: B-pattern with existence of 3 or more B lines per intercostal space; 2: severe B-pattern: coalescence of B lines or small subpleural consolidations; and 3: extensive consolidations. Source: JAMA Pediatr. 2015; 169: e151797. Available in: https://doi.org/10.1001/jamapediatrics.2015.1797.
Prevention
The best way to prevent distress is to avoid premature birth or for it to occur in the best-prepared Neonatology Units, thus favoring the intrauterine transfer of at-risk mothers and using tocolytics. Administration of antenatal corticosteroids (at least a complete cycle in pregnancies less than 34 weeks and considering its repetition if more than a week passes after its administration) and magnesium sulfate in mothers with imminent delivery less than 32 weeks are also important. In the resuscitation room: delayed clamping of the umbilical cord; the use of monitoring with preductal pulse oximetry providing initial FiO2 of 30% in those under 30 weeks and adjusting it according to saturation achieved; the use of polyethylene bags; and early application of continuous positive airway pressure (CPAP). Nasal administration with initial positive end-expiratory pressure (PEEP) of 6 cmH2O and performing T-tube resuscitation and early intermittent positive pressure may be necessary for resuscitation.
Treatment
The 2022 European consensus guidelines(3) for the management of respiratory distress indicate the administration of surfactant in infants under 30 weeks of age who have required intubation. The application of nasal CPAP with PEEP 6-8 cmH2O constitutes the recommendation for initial respiratory support in children under 30 weeks who do not require intubation, with the administration of surfactant using minimally invasive techniques (LISA: Less Invasive Surfactant Administration) being indicated if they require FiO2 higher than 30% to maintain preductal oxygen saturation of 90-94%. Thoracic ultrasound indicates early administration of surfactant with a LUS-score greater than 8 points and the need for non-invasive respiratory support. The initial dose of surfactant (poractant alfa) is 200 mg/kg, and it can be repeated (100 mg/kg) as rescue therapy 6-12 hours after the first dose.
The use of non-invasive nasal ventilation constitutes another possibility as initial respiratory management or in extubation, with synchronized modalities being of choice. High flow nasal air/oxygen is still considered a second option as initial support but can also be used as a weaner from non-invasive ventilation.
In invasive mechanical ventilation modalities, the shortest intubation time and the use of lung protection modalities are sought (guaranteed volume, pressure support modalities –PSV–, high frequency modality). Caffeine citrate (loading bolus 20 mg/kg and maintenance 5-10 mg/kg), adequate management of fluid intake, the use of hemodynamic support if necessary, the judicious need for antibiotic therapy and transfusions as well as the adequate provision of early parenteral and enteral nutrition are also key tools in management.
Chronic lung disease: bronchopulmonary dysplasia (BPD)(1,5,6)
Definition. Etiopathogenesis
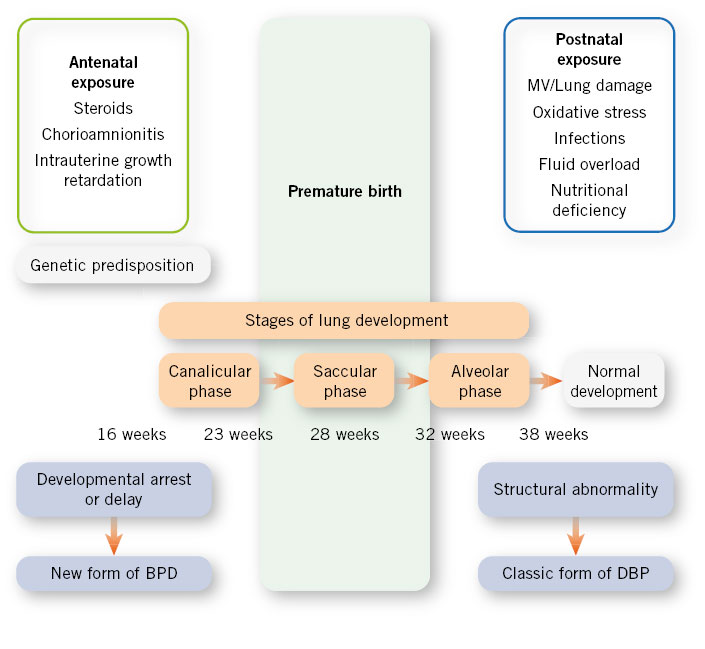
Since the origin of its description (Northway 1967), the concept has evolved from a structural pathology to a “new BPD” more related to the arrest of lung development present in extreme PTNBs. The etiology is multifactorial, influencing both genetic factors (male sex), prenatal (chorioamnionitis, intrauterine growth retardation) and postnatal (oxygen therapy, baro and volutrauma in ventilation, fluid overload, hemodynamic instability) (Fig. 3).
Figure 3. Etiopathogenesis of bronchopulmonary dysplasia (BPD). Source: An Pediatr Contin. 2011; 9: 89-97. MV: mechanical ventilation.
Pathophysiology
The need for respiratory support with oxygen therapy and mechanical ventilation in developing lungs generates an inflammatory response, interrupting alveolarization and vascular development evident in the “new” forms of BPD(5,6).
Clinical manifestations
It is characterized by complicated weaning from respiratory support, with home oxygen supplementation sometimes being necessary upon discharge. These patients are at risk of respiratory morbidity in the first 2 years of life with a greater susceptibility to respiratory infections (including respiratory syncytial virus) and episodes of wheezing and bronchospasm.
Diagnosis
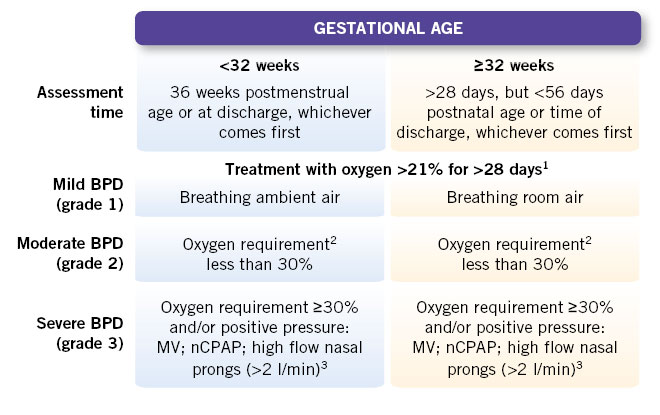
Clinical criteria and the need for respiratory support and oxygen therapy are used for its classification and diagnosis (Fig. 4).
Figure 4. National Institute Health (NIH) consensus of 2001 with later modifications that include the use of nasal cannulas. – 1Patients treated with oxygen >21% and/or positive pressure or high flow due to non-respiratory pathology (e.g.: central apneas or diaphragmatic paralysis) are not considered bronchopulmonary dysplasia (BPD) unless they develop parenchymal lung disease and show clinical signs of respiratory distress. A day of oxygen treatment >21% is considered if received for more than 12 hours a day. – 2A physiological test is required confirming the need for oxygen: oxygen reduction test. The diagnosis of moderate BPD is established if the oxygen reduction test fails, failing to maintain a saturation ≥90% breathing room air. – 3Treatment with oxygen FiO2 >30% and/or positive pressure with any non-invasive ventilation system or nasal cannula with more than 2 l/min (regardless of FiO2), without which an oxygen saturation ≥90% cannot be maintained at 36 weeks postmenstrual age or 56 days postnatal age, and it should reflect the patient’s usual therapy in the days before or after that date, not a one-time event. In these cases, BPD is considered severe (grade 3) and oxygen reduction testing is not necessary. Definition of the oxygen reduction test: indicated in newborns with BPD with a need for FiO2 less than or equal to 30% for SpO2 >90% or who require FiO2 >30% to maintain SpO2 >96%. It is performed with the newborn in a supine position for 30 minutes after feeding and with their usual oxygen needs, measuring heart rate and respiratory rate SpO2, apneas and bradycardias for 15 minutes. The test is performed by reducing the FiO2 by 2% every 5 minutes if breathing in an open chamber, or a decrease of 0.1-0.5 lpm if breathing through nasal prongs until they are removed, maintaining this withdrawal for 60 minutes. Failure of the test is considered if SpO2 80-89% is required for >5 minutes or SpO2 <80% for >15 seconds.
Modified from: Spanish Society of Neonatology. Protocols 2023.
MV: mechanical ventilation; nCPAP: nasal continuous positive pressure.
Prevention
Prenatal administration of corticosteroids at gestational age <34 weeks, use of early surfactant, adequate postnatal fluid and hemodynamic management, lung protection strategies in respiratory support, SpO2 goal 90-95% and ventilation with permissive pCO2 ranges, after first weeks, can aid in the prevention of BPD. As preventive targets, the use of caffeine has shown evidence. Other preventive targets with improvement, but without evidence, are: breastfeeding; intramuscular vitamin A if high local incidence of BPD; postnatal use of corticosteroids (DART protocol with dexamethasone) in those patients dependent on mechanical ventilation (MV); and FiO2 greater than 50% at 2-4 weeks of age.
Treatment
Treatment consists of adequate provision of fluids and nutritional support, with own or fortified donated breastfeeding being preferred. Respiratory management with non-invasive support and, in the case of MV support, high tidal volumes of 7 ml/kg will be used with a tendency towards low respiratory frequencies and high PEEP for adequate recruitment, with the PSV modality with long inspiratory times being a good choice. Other possible measures used are: thiazide diuretics in possible combination with spironolactone or specific doses of loop diuretics; occasional corticosteroids in exacerbations; and monitoring of clinical worsening in the context of possible respiratory infections with adequate prevention (prophylaxis of respiratory syncytial virus and influenza virus in epidemic periods).
Patent ductus arteriosus (PAD)(1,7,8)
Definition
It is a vascular structure that communicates, in fetal life, the main pulmonary artery with the descending aorta, producing its functional closure in the first 72 hours of life in full-term neonates and its subsequent anatomical closure.
Pathophysiology
In PTNBs there is a histological immaturity of this duct due to a smaller number of muscle fibers in its wall, together with a local environment with a greater amount of prostaglandins and prostacyclins that condition its vasodilation and greater difficulty in its spontaneous closure. Some predisposing factors are: lower gestational age, immediate respiratory distress, maternal diabetes, prepartum hemorrhage, multiple pregnancy, excessive postnatal fluid intake, administration of furosemide in the first days of life and any inflammatory situation such as infectious processes.
Clinical manifestations
PAD causes a theft of flow from the systemic circulation with an increase in it at the pulmonary level. Initially, it may remain silent with progression of auscultation to a continuous precordial murmur and palpation of bulging and full femoral and pedial pulses. It progresses to signs of left heart failure (oligoanuria, tachycardia and tachypnea, peripheral hypoperfusion, decrease in diastolic blood pressure, hepatomegaly) and respiratory worsening, with pulmonary edema. It associates increased risk of necrotizing enterocolitis, bronchopulmonary dysplasia, intraventricular hemorrhage, and death.
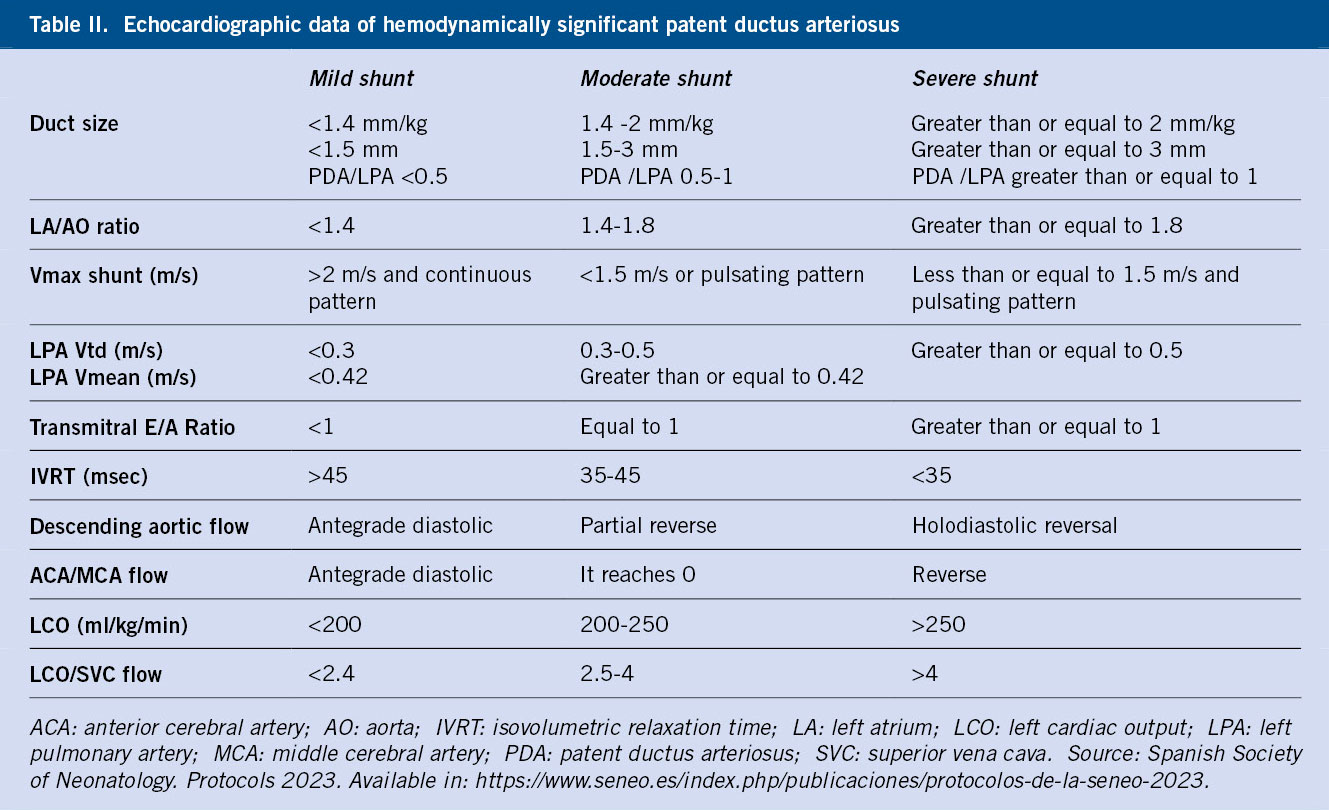
Diagnosis
It is made using echocardiographic parameters that help us specify the possibility of spontaneous closure (Table II)(1).
Treatment
Fluid restriction, adequate respiratory support with increased PEEP and, if necessary, the use of thiazide diuretics, are the therapeutic options. Treatment is chosen in those cases with hemodynamic repercussions, with ibuprofen being the choice in 3 doses, each separated by 24 hours (10-5-5 mg/kg). In case of contraindication (severe renal failure, active bleeding, thrombocytopenia <25,000, coagulopathy, severe sepsis, enterocolitis) treatment with paracetamol would be chosen for a maximum of 7 days (10-15 mg/kg/6-8 h). If it remains open, a second cycle could be administered and, if it fails, surgical closure would be required, monitoring the possibility of post-ductus ligation syndrome that causes low systemic output (need for volume, dobutamine, hydrocortisone and consider early use of milrinone)(8).
Intraventricular hemorrhage (IVH)(1,9,10)
Definition. Etiopathogenesis
Brain pathology marks the long-term prognosis of PTNBs. Its incidence is 20-25%, with an increase at lower GA. The etiopathogenesis is marked by the immaturity of the germinal matrix added to risk factors: hemodynamic instability (volume expansions, inotropic support); respiratory (alterations in ventilation and oxygenation, pneumothorax); metabolic (ionic alterations and hypoglycemia); thermal (hypothermia); hematological (anemia, transfusions); exposure to stimuli (noise, light and positioning); and improper handling.
Pathophysiology
Its pathophysiology resides in immaturity in the regulation of cerebral blood flow; so that small variations in blood pressure generate alterations in pressure-perfusion and blood volume in the germinal matrix.
Clinical manifestations
In 50% of PTNBs, the symptoms are usually silent or unnoticed, however, a severe presentation with rapid neurological and systemic deterioration is also possible.
Diagnosis(1)
Routine transcranial ultrasound controls are the essential tool (under 28 weeks to be performed at days: 1-3-7-14-weekly thereafter). Brain magnetic resonance imaging in the postmenstrual age (PMA) at term and the assessment of general developmental movements(10) are helpful tools.
The IVH classification marks three degrees of it: I = germinal matrix hemorrhage with only 10% of possible intraventricular involvement; II = intraventricular hemorrhage of 10-50% parasagittal plane; and III = intraventricular hemorrhage greater than 50% and/or ventricular dilation, and possible periventricular venous hemorrhagic infarction (PVHI; formerly grade IV in the classifications) whose greater extension or if bilateral, worsens the prognosis.
Another type of hemorrhage is located at the cerebellar level with diagnosis being made by ultrasound (mastoid window) or brain magnetic resonance imaging.
Prevention
The following are considered prevention strategies: the use of antenatal corticosteroids, maternal antibiotic therapy in case of a ruptured bag, minimal manipulation and careful hemodynamic and respiratory management, early enteral feeding, reduction of blood extractions, rapid volume expansions or unnecessary transfusions.
Complications and prognosis(9)
Advanced degrees of IVH and PVHI lead to possible motor and cognitive sequelae, epilepsy and increased mortality. Posthemorrhagic hydrocephalus due to cerebrospinal fluid (CSF) drainage obstruction due to clots presents with a bulging fontanelle and an increase in head circumference that may appear during the course of the disease. In the diagnosis, ultrasound indices are used: ventricular index, width of the anterior horn of the ventricle and the thalamo-caudate distance.
Treatment
Serial ultrasound control should be performed to monitor the possible appearance of hydrocephalus. If the ventricular index is >p97 + 4 mm, 2 lumbar punctures will be performed to evacuate a maximum of 10 ml/kg of CSF and, if there is no improvement, a ventricular access device will be placed and long-term evaluation of definitive ventricular drainage.
Early Care stimulation programs as well as follow-up of these patients is crucial.
Periventricular leukomalacia (PVL)(1,9,10)
Definition. Etiopathogenesis
White matter involvement can occur in more than 1/3 of children under 28 weeks, marking the neurodevelopmental prognosis. The risk factors for the development of this injury are the same as those for intraventricular hemorrhage, and adequate manipulation is essential.
Pathophysiology(1)
Immaturity of the preoligodendrocyte that constitutes the main cell of axonal coverage in the white matter of the PTNB makes it vulnerable to: hemodynamic vascular changes (minimal drops in blood pressure), situations of hypoxia-ischemia and any situation that generates an inflammatory cascade in said area. This can cause interruption of the normal development of myelination or local necrosis.
Clinical manifestations
At first it may be silent, hence, serial neurological assessment (general movements) is important.
Diagnosis(9)
It is made by serial transcranial ultrasound control (under 28 weeks: 1-3-14-21 days of life and, subsequently, weekly) and brain magnetic resonance imaging at term age, which must be individualized.
The classification of the severity of white matter involvement is marked by the persistence of periventricular hyperechogenicity beyond two weeks of life or a heterogeneous appearance and by the presence of cysts or indicators of volume loss of said substance (dilation of the ventricles, decrease in thickness of the corpus callosum, widening of the interhemispheric fissure, loss of thalamus volume, increase in subarachnoid space, decrease in cortical folding).
Prevention. Treatment
The same preventive factors as in IVH apply, as well as the administration of magnesium sulfate at the time of delivery.
As therapy, the important role of early stimulation and monitoring in multidisciplinary Early Care teams(10).
Necrotizing enterocolitis (NEC)
Definition. Etiopathogenesis(1,11,12)
It is a gastrointestinal emergency in PTNBs (2-7.5% in those less than 32 weeks GA). It consists of ischemic necrosis of the intestinal mucosa with severe inflammation. The most frequently affected areas are the ileum and ascending colon.
Pathophysiology
The immaturity of local defenses and intestinal function increases the proliferation of non-commensal microorganisms, which may favor bacterial translocation, inflammation and the release of cytokines.
Clinical characteristics(12)
They usually appear in the first or second week of life. The condition can be slowly progressive or sudden and fulminant. The presentation is marked by a systemic picture (hemodynamic and thermal instability, respiratory failure and lethargy) and abdominal manifestations (digestive intolerance, bloody stools, abdominal examination with pain and distention, erythema of the wall, crepitation and induration).
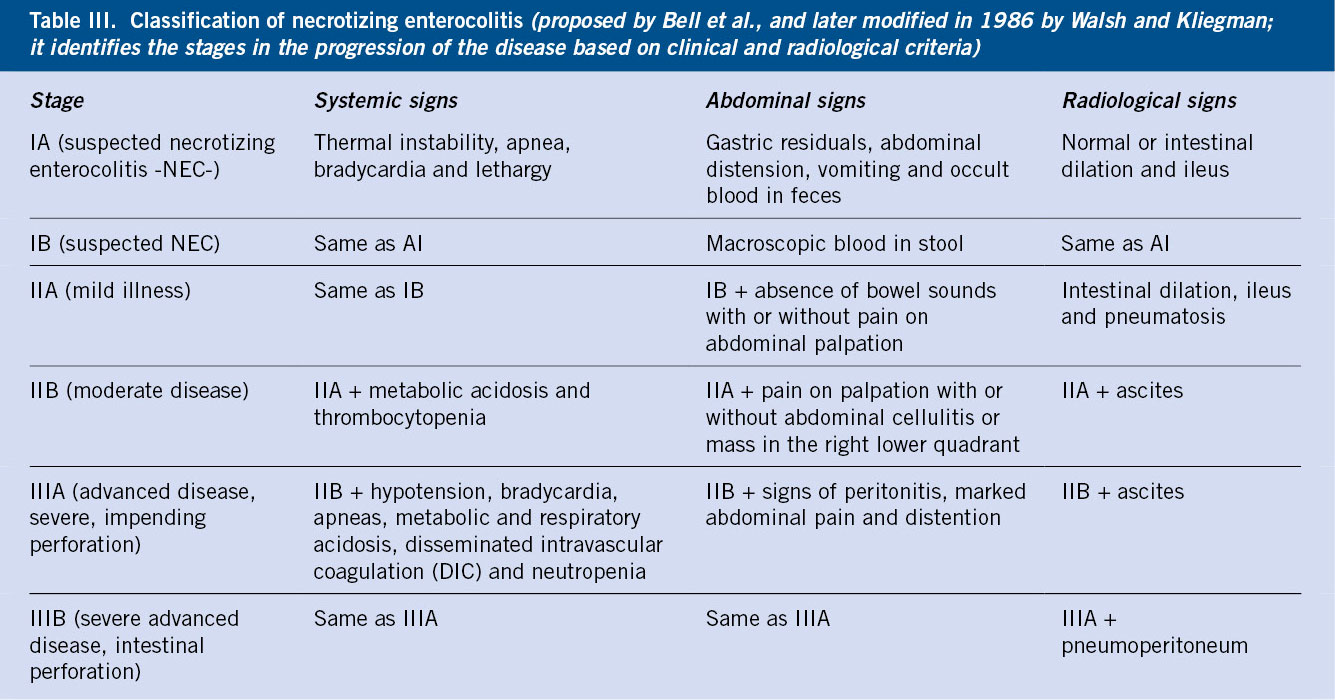
Diagnosis(11,13)
The clinical suspicion is confirmed by serial radiological studies (Table III).
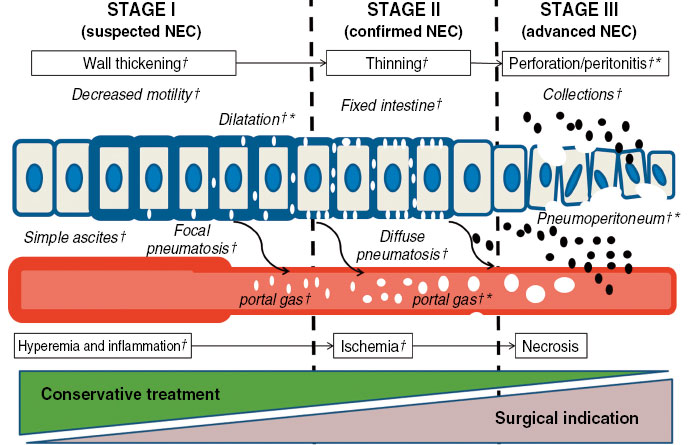
Ultrasound allows us to anticipate the diagnosis of perforation, being superior to simple radiography in the diagnosis of ascites or collections(13) (Fig. 5).
Figure 5. Progression of the pathological process in necrotizing enterocolitis (NEC). (†) Marks signs detectable by ultrasound and (*) the signs detectable by radiography. Source: An Pediatr (Barc). 2020; 93: 411-9.
Laboratory findings that may be present include: anemia, thrombopenia, neutropenia with left shift, increased C-reactive protein, hyperglycemia, hyponatremia and metabolic acidosis.
Prevention(1)
Prenatal administration of corticosteroids, delayed cord clamping, early feeding with breast milk (own or donated). Risk factors include: prolonged use of antibiotic therapy, as well as the use of antacids (anti-H2 or proton pump inhibitors).
Treatment(1,11)
Treatment includes: digestive rest with provision of parenteral nutrition and intestinal decompression through a nasogastric tube, maintaining adequate hydroelectrolyte control (considering third space losses), ensuring respiratory and hemodynamic support, as well as transfusions of blood products if necessary, analgesia and initiation of broad spectrum antibiotic therapy (the most frequently isolated germs are: E. coli, Enterobacter, Klebsiella and coagulase negative Staphylococcus).
Up to 25-50% may require surgical treatment, with the aim of resecting the necrotic intestine and exteriorizing the viable ends through ostomies. In cases of generalized intestinal involvement, a second exploratory laparotomy may be required after 24-48 hours. The use of peritoneal drainage as a surgical option is controversial. Short intestine is a possible long-term complication, requiring complex nutritional management in these patients.
Retinopathy of prematurity (ROP) (Fig. 6)(1,14,15)
Figure 6. Staging of retinopathy of prematurity. Source: Arch Soc Esp Oftalmol. 2013; 88: 231-6.
Definition. Etiopathogenesis
It is a disease that affects the normal development of the blood vessels in the retina of the PTNB and is the main preventable cause of childhood blindness. Etiopathogenic factors include: prematurity, exposure to high oxygen concentrations and fluctuation of its levels, infections, transfusions, use of erythropoietin, bronchopulmonary dysplasia and intracranial hemorrhage.
Pathophysiology
PTNBs are born with an immature retina in terms of its vascularization, which is completed around 36-40 weeks. An imbalance of angiogenic factors such as insulin-like growth factor (IGF) and vascular endothelial growth factor (VEGF) occurs, resulting in the appearance of neovessels and fibrovascular tissue that can evolve into traction with retinal detachment.
Classification. Evolutionary stages
It is classified according to its location (zones), extension (time meridians) and severity (degrees). The retina is divided into three zones concentric to the optic nerve: zone I (innermost), zone II and, finally, zone III (most peripheral). At the same time, the retina is subdivided into hourly meridians (0 to 12) to identify the affected areas. The severity of the impact is divided into:
• Non-proliferative phases (ROP grade 1: presence of demarcation line between the vascular and avascular retina; and ROP grade 2: thickening of the demarcation line: ridge).
• Proliferative phases (ROP grade 3: extraretinal vascularization; ROP grade 4: subtotal retinal detachment; and ROP grade 5: total retinal detachment).
• “Plus disease” is in which there is dilation and tortuosity of the retinal vessels of the posterior pole in at least two quadrants, and/or pupillary rigidity.
Prevention
The following recommendations aim to prevent ROP: Avoidance of hyperoxia or fluctuations in oxygen levels, optimizing oxygen saturation ranges at 90-95%, as well as universal screening between the fourth and sixth week of postnatal life in all PTNBs <1,500 g at birth and/or gestational age ≤30 weeks and in those with weight between 1,500 and 2,000 g at birth or gestational age >30 weeks at risk who have received supplemental oxygen for several days.
Treatment(1,15)
• ROP with indication for treatment: any grade of ROP in zone I with plus disease, grade 3 ROP in zone I without plus disease and grade 2 or 3 ROP in zone II with plus disease.
• ROP with indication for close monitoring: grade 1 or 2 ROP in zone I without plus disease and grade 3 ROP in zone II without additional illness.
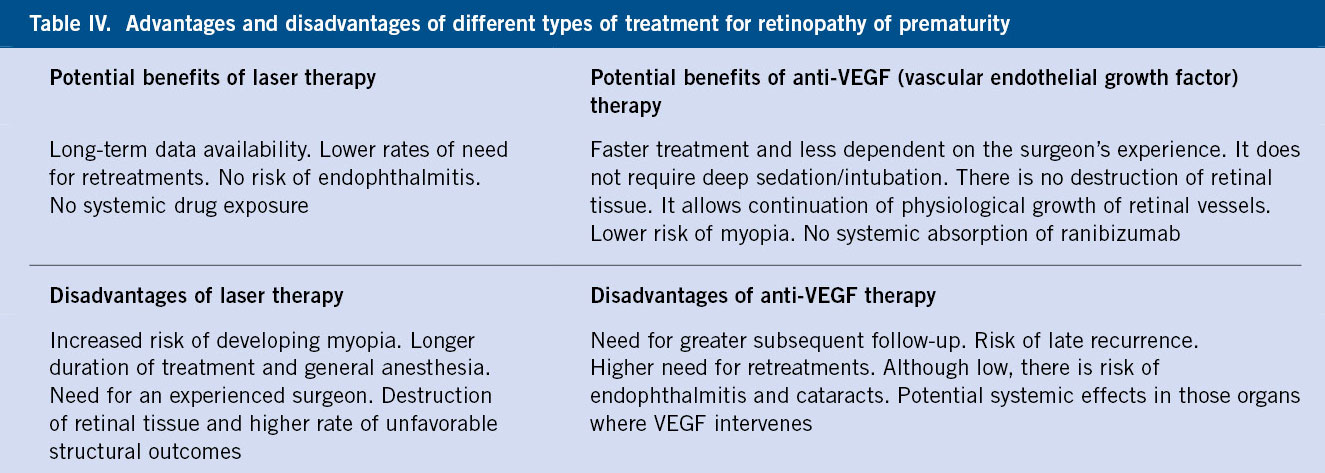
The treatments used are laser therapy on the avascular retina and anti-VEGF monoclonal antibodies (bevacizumab, ranibizumab, aflibercept), more indicated if central ROP (Table IV).
Anemia of prematurity
Definition. Pathophysiology(1,16)
It is a more pronounced degree of physiological anemia that occurs in the neonatal period within the first 8-12 weeks of age, reaching the minimum hemoglobin (Hb) level earlier in the PTNB than in term infants.
Clinical picture
Manifestations include: dyspnea during feedings, weight stagnation, tachycardia, increased apnea or oxygen requirement and metabolic acidosis.
Treatment and prevention(16)
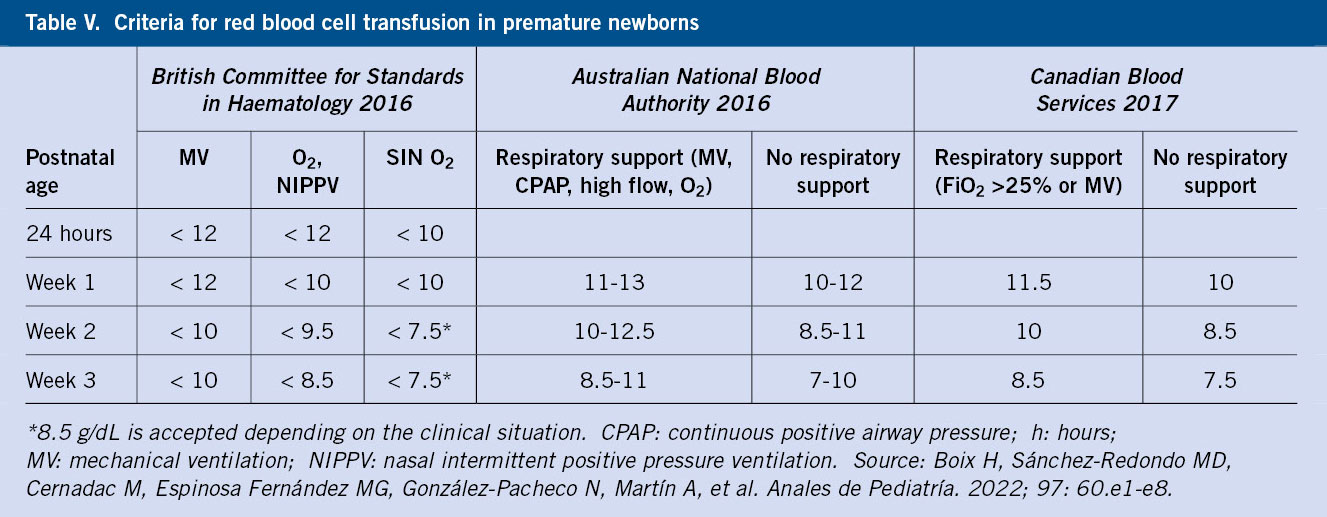
The preventive measures are: late cord clamping, reducing extractions and the use of micromethods. It is recommended to supplement with iron in the form of ferrous sulfate (2-4 mg/kg/day) for children under 1,500 g and/or under 32 weeks of age who are breastfed, from one month of age to one year of chronological age or until the introduction of red meat and cereals supplemented with iron. There are different international guidelines for transfusion criteria. The administration volume varies between 10-20 ml/kg with universally leukodepleted red blood cells and, in addition, irradiated in those <1,500 g, as well as the use of adult fractionated units in order to reduce the number of donors (Table V).
Jaundice(17)
It is the yellowing of skin due to the deposition of bilirubin. It is the most frequent cause of hospital readmission of PTNBs. The risk factors are: liver immaturity; difficulties in feeding, being more vulnerable with a greater risk of brain damage; and kernicterus, due to the immaturity of the blood-brain barrier and a lower amount of circulating albumin carrier.
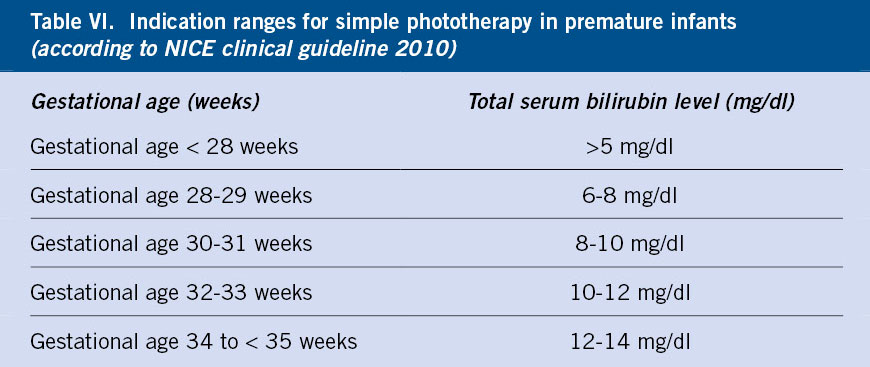
Treatment is based on adequate nutrition, phototherapy and exchange transfusion, if necessary. For newborns older than 35 weeks, the updated 2022 guidelines from the American Academy of Pediatrics will be followed. In the case of children under 35 weeks, the data are more limited and the established recommended treatment levels are based on expert consensus (Table VI).
Role of the Primary Care pediatrician
Premature newborns under 32 weeks require long periods of admission to Neonatology Units with multidisciplinary management and monitoring of possible complications that may arise. At the time of discharge, it is important to explain to parents the difference between chronological and postmenstrual age, as well as to provide the necessary follow-up appointments at the hospital level. The role of the Primary Care pediatrician is key in the pediatric progress of these patients, in the administration of the appropriate vaccination schedule and in the joint assessment with the follow-up in Neonatology and other pediatric specialties (Pulmonology, Gastroenterology, Endocrinology, etc.).
Conflict of interest
There is no conflict of interest in the preparation of the manuscript. Declaration of interests: none.
Bibliography
The asterisks show the interest of the article in the authors’ opinion.
1.*** Protocolos Sociedad Española de Neonatología. 2023. Protocols Spanish Society of Neonatology. 2023. Available in: https://www.seneo.es/index.php/publicaciones/protocolos-de-la-seneo-2023.
2. Madar J, Roehr C, Ainsworth S, Ersdal H, Morley C, Rüdiger M, et al. European Resuscitation Council Guidelines: Newborn resuscitation and support of transition of infants at birth. Resuscitation. 2021; 161: 291-326.
3.*** Sweet D, Carnielli VP, Greisen G, Hallman M, Klebermass-Schrehof K, Ozek E, et al. European consensus guidelines on the management of respiratory distress syndrome: 2022 update. Neonatology. 2023; 120: 3-23.
4. Brat R, Yousef N, Klifa R, Reynaud S, Shankar S, De Luca D. Lung. Ultrasonography score to evaluate oxygenation and surfactant need in neonates treated with continuous positive airway pressure. JAMA Pediatr. 2015; 169: e151797. Available in: https://doi.org/10.1001/jamapediatrics.2015.1797.
5. Pérez G, Navarro M, Andrés A. El prematuro con enfermedad pulmonar crónica/displasia broncopulmonar: seguimiento. The preterm with chronic lung disease/bronchopulmonary dysplasia: follow-up. An Pediatr Cont. 2011; 9: 89-97.
6. Jensen E, Dysart K, Gantz M, McDonald S, Barnat N, Keszler M, et al. The Diagnosis of Bronchopulmonary Dysplasia in Very Preterm Infants: An Evidence-based Approach. American Journal of Respiratory and Critical Care Medicine. 2019; 200: 751-9.
7.*** Van Laere D, Van Overmeire B, Gupta S, El Khuffash A, Savoia M, McNamara P, et al. Application of NPE in the assessment of a patent ductus arteriosus. Pediatric Research. 2018; 84: 46-56.
8. Giesinger R, Bischoff A, McNamara P. Anticipatory perioperative management for patent ductus arteriosus surgery: Understanding postligation cardiac syndrome. Congenital Heart Disease. 2019; 14: 311-6.
9.*** García-Alix A, Arnáez J. Patología cerebral del prematuro en: Neurología Neonatal de un vistazo. Brain pathology of the premature in: Neonatal Neurology at a glance. Madrid: Cabeza de Chorlito; 2022. p. 154-64.
10. García-Alix A, Arnáez J. Los movimientos generales del neonato y del lactante. General movements of the neonate and infant. Barcelona: Nene Foundation and EDISEBEN. 2022. Available in: https://www.neurologianeonatal.org/images/recursos/libros/Movimientos-generales-en-el-neonato-y-el-lactante.pdf.
11.*** Theresa C, Antonella D, de Ville de Goyet Jean. New Nutritional and Therapeutical Strategies of NEC. Curr Pediatr Rev. 2019; 15: 92-105.
12. López Herrera MC. Enterocolitis necrosante. Necrotizing enterocolitis. In: Hernández MC. Treaty of Pediatrics. Ergon Editions; 2001. p. 270-1.
13. Oulego-Erroz, Terroba-Seara S, Alonso-Quintela A, Jiménez-González A, Ardela-Días ErickI. Ecografía a pie de cama en el diagnóstico precoz de la enterocolitis necrosante: Una estrategia para mejorar el pronóstico. Bedside ultrasound in the early diagnosis of necrotizing enterocolitis: A strategy to improve prognosis. An Pediatr. 2020; 93: 411-3.
14. Ferrer Novella C, González I, Pueyo V, Martínez R, Galdós M, Peralta J, et al. Screening program for retinopathy of prematurity in Spain. Arch. Soc. Esp. Oftalmol. 2013; 88: 184-8.
15.*** Dammann O, Hartnett ME, Stahl A. Retinopathy of prematurity. Dev Med Child Neurol. 2023; 65: 625-31.
16.*** Boix H, Sánchez-Redondo MD, Cernada M, Espinosa MG, González N, Martín A, et al. Recomendaciones para la transfusión de hemoderivados en neonatología. Recommendations for transfusion of blood products in neonatology. An Pediatr. 2022; 97: 60.e1-e8.
17. González-Valcárcel M, Raynero RC, Caballero SM. Ictericia neonatal Temas de FC. Neonatal jaundice as parto f Continuous Formation Topics. Pediatr Integral. 2019; XXIII: 147-53. Available in: https://www.pediatriaintegral.es/publicacion-2019-05/ictericia-neonatal-2/.
Recommended bibliography
– Protocolos Sociedad Española de Neonatología. Protocols of the Spanish Society of Neonatology. 2023. Available in: https://www.seneo.es/index.php/publicaciones/protocolos-de-la-seneo-2023.
Reading the protocols of the Spanish Society of Neonatology updated in 2023 is advisable, as they collect in a clear and updated way the pathologies and management in the area of Neonatology.
– Sweet D, Carnielli VP, Greisen G, Hallman M, Klebermass-Schrehof K, Ozek E, et al. European consensus guidelines on the management of respiratory distress syndrome: 2022 update. Neonatology. 2023; 120: 3-23.
This article details the current changes in the diagnosis and management of neonatal respiratory distress; focusing on the characteristics of respiratory support in invasive and non-invasive mechanical ventilation, as well as in the decision to administer surfactant.
– García-Alix A, Arnáez J. Patología cerebral del prematuro en: Neurología Neonatal de un vistazo. Brain pathology of the premature in: Neonatal Neurology at a glance. Madrid: Cabeza de Chorlito; 2022. p. 154-64.
It constitutes a manual of management algorithms in the neurological pathology of newborns, with clear and quick schemes that help guide diagnosis and therapeutic action.
| Clinical case |
|
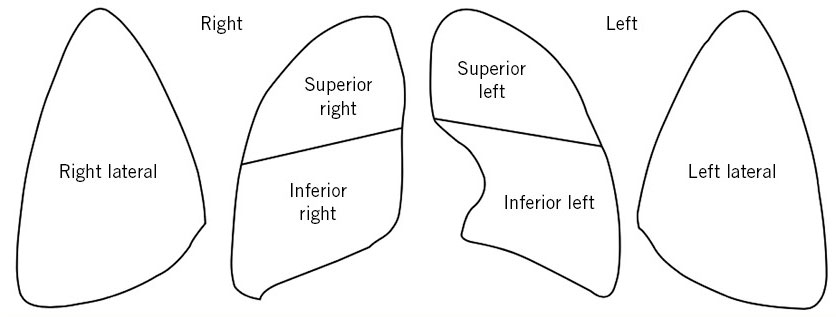
Reason for admission A preterm newborn of 35+1 weeks GA is admitted from the delivery room due to immediate distress. Family background Mother: 39 years old; Number of pregnancies/gestational losses/live births: 5/2/3. Blood group: A Rhesus positive. Maternal history of severe L4-L5 disc disease. Personal history Controlled pregnancy. Antenatal serologies: immune to rubella, with the rest being negative (HIV, HBV, HCV, toxoplasma and syphilis). Normal and concordant ultrasounds. Rectovaginal culture negative for Streptococcus agalactiae. Maternal gestational diabetes from week 26, treated with insulin. As a background, the mother required admission at 32 weeks of gestation to control pain due to disc disease, where she received lung maturation with corticosteroids. Labor was induced at 35+1 weeks due to maternal disc pathology. Membranes were ruptured for 9 hours with clear liquid. Caesarean section was performed due to non-progression of labor. There was no maternal fever or antibiotic therapy during delivery. He was born crying and with a good tone; Apgar score: 9/10; Arterial cord pH: 7.28. Newborn weight: 2,350 g (39th centile). Examination Initial preductal SpO2 90% with FiO2 room air. He presents good general condition, well perfused but with polypnea, audible moan without stethoscope and subcostal indrawing. In the cardiopulmonary auscultation he has good bilateral air entry and a grade I/VI systolic murmur. The abdomen is soft, depressible without masses or visceromegaly and he has palpable testes in the scrotal bags. Hips are normal on examination. Neurologically, he is responsive, with mild axial hypotonia. Clinical progress Upon admission, the patient was placed in a neutral thermal environment and oculohemorrhagic prophylaxis was administered. Peripheral venous access was obtained and he was started on iv fluids with glucose and calcium according to basal needs. Respiratory support was started with nasal CPAP, PEEP 6 cmH2O with initial FiO2 21%. In the first two hours of life he required an increase in FiO2 needs up to 28%, with venous gas control of pH: 7.33; pCO2: 60 mmHg; EB: -2.7 mmol/L. Pulmonary ultrasound control was performed at the side of the incubator with an alveolointerstitial pattern in both the upper and lower right and left fields and in the lateral fields (the image repeated in the 6 fields is attached) (Fig. 7). Figure 7. LUS-score grading scheme in the 6 quadrants and the image in each of them. Given this finding and persistence of respiratory distress, a dose of intratracheal surfactant (200 mg/kg) was administered. He showed improvement in the respiratory pattern with a subsequently decrease in FiO2 to 21% and allowing withdrawal of respiratory support on the second day of life. During his stay he maintained normal hemodynamic constants. Glycemic controls due to a history of maternal diabetes were within normal limits. Given persistence of distress, a blood culture was collected and ampicillin and gentamicin antibiotics administered, which were withdrawn after 48 hours due to a negative preliminary blood culture and optimal clinical progress. Transcranial ultrasound control was performed upon admission, with results within normal limits. Enteral tolerance began in the first 24 hours with mother´s own breast milk, initially through an orogastric tube and after withdrawal of respiratory support with direct latching to the breast and suction. At 36 hours of age, the patient presented a maximum bilirubin level of 12 mg/dl, requiring treatment with simple continuous phototherapy (American Academy of Pediatrics charts). Favorable clinical progress followed with maximum weight loss of 8% and subsequent ascending weight curve, allowing discharge home at 5 days of age; with clinical consultation appointment in the first month of life, where he showed adequate weight gain and good feeds.
|
 Prevalent pathologies in prematurity
Prevalent pathologies in prematurity