|
| Topics on Continuous Training |
J. de la Flor i Brú
Health Care Center “El Serral”. ABS Sant Vicenç dels Horts-1. DAP Baix Llobregat-Litoral. ICS. Barcelona
| Abstract
Otitis media is one of the most frequent diagnoses in Primary Care (PC). Acute otitis media (AOM) is mainly caused by pneumococcus and non-typeable Haemophilus influenzae. The specific clinical symptomatology is otalgia or its equivalents. Otoscopy should show tympanic bulging or bluish or yellowish tympanic discoloration, or a combination of hyperemia and dullness. Treatment should begin with proper pain relief. The use or not of antibiotics (ATB) should be based on a correct diagnosis. Watchful waiting may be an initial choice in children older than 2 years, without risk factors. High-dose amoxicillin is the first choice treatment. Suppurative AOM with perforation should always be treated with ten days of antibiotics. Recurrent acute otitis media (RAOM) and chronic otitis media with effusion (COME) are the most prevalent chronic diseases in primary pediatric care. RAOM is defined by the presence of 3 or more episodes in less than 6 months, or 4 or more in less than 12 months, provided that the last episode occurred in the last 6 months. COME is defined as the presence of bilateral effusion for more than 3 months or unilateral for more than 6 months. RAOM must be initially managed with intervention on predisposing factors. Surgery should be the last resort. COME should be diagnosed with objective detection of middle ear effusion (tympanometry). Persistent conductive hearing loss greater than 30 decibels must be considered amenable to treatment with tympanostomy tubes. Otorrhea in carriers of tympanostomy tubes must be treated with topical ciprofloxacin. External otitis is produced by moist in external auditory conduct. Hygienic counseling and topical antibiotic will suffice in a vast number of cases. |
| Resumen
La otitis media es uno de los diagnósticos más frecuentes en Atención Primaria (AP). La otitis media aguda (OMA) es causada fundamentalmente por neumococo y Haemophilus influenzae no capsulado. La sintomatología clínica específica es la otalgia o sus equivalentes. La otoscopia debe mostrar abombamiento timpánico o, en su defecto, coloración La otitis media aguda de repetición (OMAR) y la otitis media crónica con exudado (OMEC) son los problemas crónicos más frecuentes en la consulta del pediatra de AP. Se define como OMAR: la presencia de 3 o más episodios de OMA en 6 o menos meses, o 4 o más en 12 o menos meses, siempre que el último de los episodios se haya producido en los últimos 6 meses. Se define como OMEC: la presencia de exudado bilateral más de 3 meses o unilateral más de 6 meses. La OMAR debe ser manejada con la intervención sobre los factores predisponentes. La cirugía debe ser el último recurso. La OMEC debería diagnosticarse basándose en técnicas objetivas de detección de exudado de oído medio (impedanciometría). Una repercusión funcional con pérdida de más de 30 decibelios es significativa y debe considerarse como susceptible de tratarse con drenaje transtimpánico. La otorrea en portadores de tubos de drenaje debe tratarse con ciprofloxacino tópico. La otitis externa se produce generalmente por exceso de humedad en el conducto auditivo. Las normas higiénicas y un tratamiento antibiótico local serán suficientes en la mayoría de casos. |
Key words: Otitis media; Otoscopy; Impedanciometry; Mastoiditis; Recurrent otitis media; Chronic otitis media; Conductive hearing loss; Tympanostomy tubes; External otitis.
Palabras clave: Otitis media; Otoscopia; Impedanciometría; Mastoiditis; Otitis media persistente; Otitis media de repetición; Otitis media crónica; Hipoacusia de transmisión; Drenaje transtimpánico; Otitis externa.
Pediatr Integral 2022; XXVI (6): 353 – 368
OBJECTIVES
• To understand the importance of differentiating otitis media from acute otitis media.
• To present the clinical criteria for diagnosing otitis media.
• To be aware of the diagnostic techniques to objectify effusion from the middle ear.
• To know the indications for expectant management without the initial use of antibiotics in acute otitis media.
• To recognize the need to use narrow-spectrum antibiotics in the initial treatment of acute otitis media.
• To identify the complementary diagnostics means to objectively assess the functional impact of chronic otitis media.
• To know the management and referral criteria for recurrent acute otitis media and chronic otitis media.
Upper respiratory tract infections-2: otitis media (etiology, symptoms and diagnosis; complications and treatment); recurrent acute otitis media and chronic otitis media; external otitis
Otitis media (etiology, symptoms and diagnosis; complications and treatment)
Introduction and epidemiology
The epidemiological importance of otitis media in PC Pediatrics is given by its frequency, by the consumption of health resources that it entails and, possibly, by its potential repercussions on the child’s development and learning.
Acute otitis media (AOM) is the most frequent diagnosis in a PC consultation after the common cold and well-child health check-ups. 80% of 3-year-olds have experienced at least one episode, and 33% have had 3 or more attacks. The peak incidence is between 6-18 months. This pathology generates a large number of spontaneous clinical visits and check-ups, and an incalculable expense derived from the often unnecessary use of drugs, referrals and surgical procedures.
Definitions
It is necessary to exactly define the different clinical pictures and to precisely recognize which entity we are referring to.
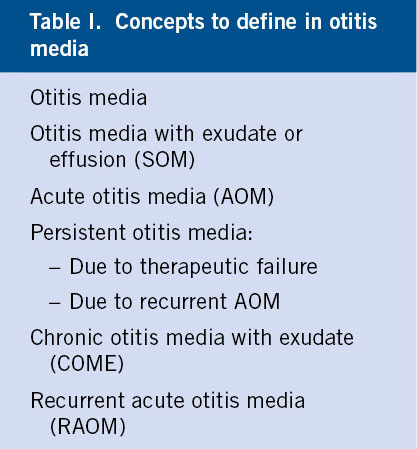
It is very convenient to clarify the terms with which we will refer to the different variants of this disease, given that there is great confusion in their use(1) (Table I).
Otitis media is defined as: inflammation of the mucosa of the middle ear, generally accompanied by the presence of fluid in such cavity. The middle ear is an air cavity in which the existence of any liquid (be it exudate, transudate, pus or blood) is always pathological.
If there are acute clinical symptoms attributable to this presence of fluid, we speak of acute otitis media (AOM).
If there are no acute clinical symptoms, otitis media is called secretory otitis media, with effusion or exudate (SOM). It is usually an evolutionary post-acute infection event and is generally related to the presence of cytokines and other inflammatory mediators in the middle ear. It presents with a transmission hearing loss of an average of 50 dB.
This situation has been classically known as “serous otitis”, a term that is currently in disuse, since it presupposes the sterility of said effusion, when in fact germs are cultivated in 20-30% of cases, with a higher prevalence of viruses. Using PCR techniques, germs are found much more frequently. These germs can be structured by covering the mucosa of the middle ear in the form of a film or biofilm, a mucoid substance with a polysaccharide matrix that protects bacteria from antibiotic activity and hides them from the immune system.
If the effusion lasts more than 3 months and is bilateral, it is called otitis media with chronic effusion (OMCE). If the effusion is only unilateral, it must last more than 6 months to be classified as chronic.
We may speak of persistent otitis media in two situations:
• When the acute symptomatology (earache and/or fever) persists beyond 48-72 hours in the course of antibiotic treatment (therapeutic failure).
• When a new acute episode occurs within 14 days of completion of antibiotic treatment for a previous episode (recurrent AOM). In these two situations, it is more likely that the same germ isolated in the initial tympanocentesis will be cultured. Beyond this limit, a new acute episode is more often a reinfection or recurrence due to another germ than a recurrence due to the same one, and should be considered a differentiated attack from the previous one(2).
If a child experiences 3 or more episodes of AOM in 6 or fewer months, or 4 in 12 or fewer months, as long as the last one occurred in the last 6 months, we will say that he or she suffers from recurrent acute otitis media (RAOM).
Etiology
In our environment, AOM must be fundamentally considered a disease caused by pneumococcus and secondarily by non-typable Haemophilus influenzae. Other germs (Moraxella, streptococcus, staphylococcus) are of secondary importance.
Knowing the bacterial flora that causes AOM makes it necessary to carry out studies in which the middle ear secretion obtained by tympanocentesis is cultured. Cultures of exudate obtained from the external auditory canal from suppurative AOM with perforation are not useful and will invariably show saprophytic flora and, especially, pseudomonas. Most of these studies come from the USA(3), and show coincidence in the order of frequency of the responsible bacteria: pneumococcus, non-typable Haemophilus influenzae (HI) (with a similar incidence in all ages) and Moraxella catarrhalis. At a great distance, Streptococcus pyogenes and staphylococcus. Alloiococcus otitidis causes up to 9% of AOM cases. Another emerging pathogen is Turicella otitidis. The few analogous studies carried out in Spain show the almost total absence of Moraxella, so for the practical purposes of proposing an empirical treatment, only pneumococcus and HI will be considered. Streptococcus is isolated more frequently in spontaneous otorrhea and in those older than 5 years, and Staphylococcus (with a growing incidence of community-acquired methicillin-resistant/MRSA) in otorrhea in children with drains. In environments with high coverage of pneumococcal conjugate vaccination, there has been a shift in etiological frequencies, with greater importance of HI(4). Up to 30% of cultures show no bacteria. In many of them, various respiratory viruses are isolated, some of which seem to induce subsequent bacterial superinfection (rhinovirus, respiratory syncytial virus, adenovirus, influenza), but their primary etiological role could account for 10% of all cases(5,6). The association of RSV bronchiolitis-otitis media is very frequent. Virus-bacteria coinfection is an added risk factor for therapeutic failure in antibiotic treatment, since viruses favor bacterial adhesion to the respiratory epithelium and alter the pharmacokinetics of ATB. It is relevant to highlight the frequent association between RSV and HI, and between influenza virus and pneumococcus.
Pathogeny
Viral infection of the nasopharynx favors Eustachian tube dysfunction, a phenomenon that combines the presence of negative pressure in the middle ear with difficulty in draining secretions into the nasopharynx.
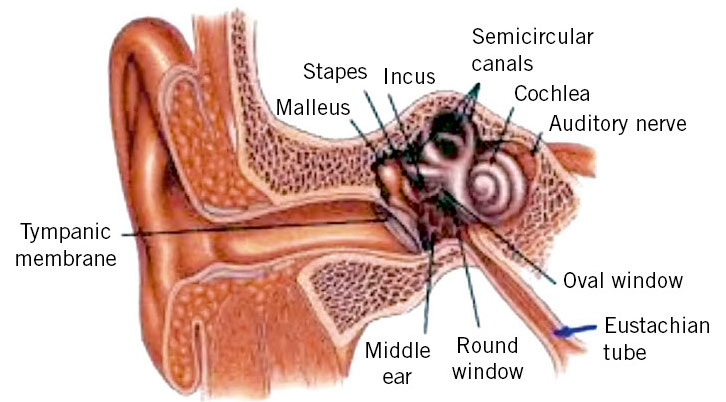
AOM is a disease related to the common cold, of which it is a common complication. It is much more common in the first 3 years of life (with a peak between 6-20 months), a time when most respiratory infections occur, especially in school children. To understand the pathogenesis of otitis media, it is necessary to know the functioning of the Eustachian tube. Its main function (Fig. 1) involves the ventilation of the middle ear with air that comes from the nasopharynx, since the balance of air pressure between the external auditory canal and the middle ear favors correct hearing.
Figure 1. Eustachian tube anatomy.
This balance is achieved with the intermittent opening of the tube with each swallowing movement, by the action of the tensor veli palatini muscle. This function is less effective in children than in adults, due to insufficient innervation, which leads to a higher frequency of negative pressures in the middle ear. This would justify the crying of the infant during pressure changes in a flight: the positive pressure of the air opens the tube and rebalances the pressure in the middle ear. The other two important functions of the tube are the protection of the middle ear from secretions coming from the nasopharynx (which during swallowing can penetrate to the isthmus or narrowing that separates the cartilaginous part of the tube from the bony part, but not to the middle ear), and drainage of mucosal secretions from the middle ear into the nasopharynx. The obstruction of the tube by mucus involves the initiation of a complex mechanism of inflammation in the middle ear. Viral infection of the upper respiratory tract also causes toxicity to the respiratory epithelium, causing ciliary dyskinesia in the Eustachian tube (Eustachian tube dysfunction, also known as ototubaritis, ETD), with impaired normal clearance of secretions. Tympanometric recordings show ETD in 75% of common cold cases. The accumulation of mucus in the tube, with the resulting obstruction, involves absorption of O2, CO2 and other gases, due to the rich vascularization of the middle ear, which generates negative pressure in the middle ear, with the net result of transudate of liquid from the mucosa, aspiration of nasopharyngeal secretions and ease for bacterial superinfection. This phenomenon is favored in the first year of life by the rectilinear conformation of the tube and its shorter length and, at any age, by passive or active smoking and allergic rhinitis. There is no otitis media without ETD.
Clinical manifestations
Otalgia or its equivalents in infants (irritability, pinna traction) is the capital sign and the one with the greatest specificity. AOM can be accompanied by other totally non-specific symptoms (fever, vomiting, diarrhea) (Table II).
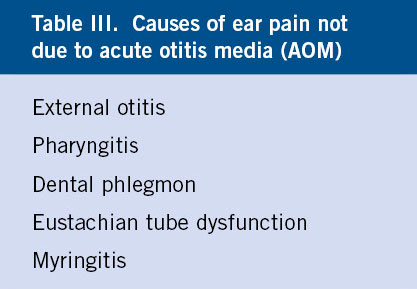
AOM usually appears a few days after the onset of a common cold, but it is not unusual for it to do so suddenly, without apparent prior catarrhal signs or when the respiratory infection already seemed to have resolved. The most specific symptom is otalgia, in the absence of which or its equivalents, AOM should not be diagnosed. However, its presence is not pathognomonic of AOM (Table III) and may be due to: external otitis, radiating pain from pharyngitis, dental phlegmon, ETD with negative pressure in the middle ear or myringitis.
It is more frequently of nocturnal appearance, since the change of position of the child when lying down favors a greater pressure of the effusion on the tympanic membrane. It can be very intense and is a frequent reason for visiting the emergency services at night. Fever is inconstant (30-60%), but can be high, especially in infants. Another symptom, less specific, but with a high predictive value, is irritability in infants who sometimes pull the ears down or rub them with their hands. It is considered equivalent to otalgia. Vomiting and/or diarrhea, in children under 3 years of age, frequently accompany AOM, but they are totally non-specific. When the tympanic membrane is perforated, discharge is visualized, which may be purulent or bloody. Perforation is usually followed by lessening or disappearance of pain. The abundant irrigation of the tympanic membrane favors rapid healing, sometimes in 24-72 hours, which can lead to a new accumulation of secretions and the reappearance of acute symptoms. Hearing loss in older children is a sign that frequently accompanies AOM and, if it is short-lived, has great specificity. Although pneumococcal AOM was classically considered to have a more acute presentation and that caused by HI tended to have a slower onset and progression, it is currently considered that the clinical picture does not allow differential assessments regarding the etiology.
Diagnosis
It is difficult, given that the symptoms can be very nonspecific, otoscopy is frequently performed in suboptimal conditions, and its interpretation is highly subjective.
Despite being one of the most frequent diagnoses that a PC pediatrician makes, the diagnosis of AOM is difficult, since it depends on the combination of clinical symptoms, sometimes nonspecific, and the adequate visualization and interpretation of tympanic membrane abnormalities by practicing otoscopy. Otoscopy is a difficult examination and subjective assessment, so it must be performed in the best conditions, with the child properly immobilized, using the widest speculum that the ear canal allows, with the absence or small amount of earwax and using an adequate light source, since when portable otoscopes are used, moderately worn batteries show images of tympanic hyperemia. The presence of obstructive cerumen (possible cause of chronic cough) is one of the most frequent problems encountered by the PC pediatrician when performing a correct otoscopy. Direct cleaning with curettes (metal or plastic) is done blindly, with the risk of causing injuries to the ear canal. Direct lavage without prior softening is often painful, usually ineffective, and must be done with absolute certainty of tympanic integrity. Pre-wash softening requires several days, which invalidates it as a method for diagnosing acute symptoms. Curettes with a built-in light source (Fig. 2) allow guided removal and immediate visualization of the tympanic membrane in more than 50% of cases where cerumen initially prevented it.
Figure 2. Luminous curette for guided removal of earwax.
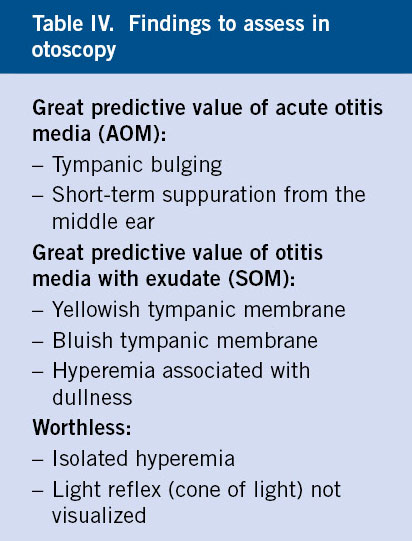
It is essential to make a brief reminder of the basic aspects of otoscopy (Table IV):
• The normal tympanic membrane is slightly concave and pearly gray or pale pink in color, being slightly transparent and allowing some visualization of the middle ear structures.
• It is especially difficult to assess in the neonatal period, due to the narrowness of the duct and the opacity of the tympanic membrane.
• Isolated hyperemia without other accompanying signs (bulging, dullness, blue or yellow discoloration) has no value, especially if the child cries, and may simply mean myringitis (inflammation of the tympanic membrane), which frequently accompanies a viral catarrhal process, without associated effusion in the middle ear. Myringitis can be an initial phase of AOM, so the child who presents it must be followed, or observed in the resolution phase of an AOM. The bullous form (bullous myringitis) is a form of presentation of AOM, with the same etiology, although in this case a possible additional etiological agent is Mycoplasma pneumoniae.
• Lack of visualization of the light reflex has no value.
• Blue or yellow color is suggestive of otitis media. The bluish eardrum is seen more frequently in SOM, and yellow in AOM, but this rule is not pathognomonic.
• Hyperemia associated with dullness is suggestive of otitis media.
• White patches in the membrane usually translate chronic or scarring processes (tympanosclerosis).
• Marked tympanic retraction indicates negative pressure in the middle ear and is suggestive of SOM.
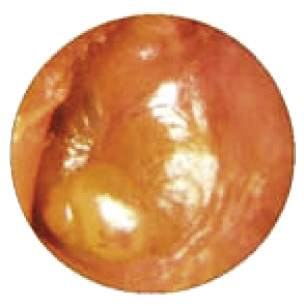
• Tympanic bulging (Fig. 3) (exceptionally present in OME) and the presence of acute suppuration from the middle ear are almost pathognomonic of AOM. Bulging is considered the otoscopic sign with the highest positive predictive value for AOM.
Figure 3. Tympanic bulge.
• The classic “tragus” or “pinna” pain signs have no value in the diagnosis of AOM. Instead, they are highly suggestive of external otitis.
• If these otoscopic examinations are accompanied by acute clinical symptoms, and fundamentally otalgia or its equivalent of irritability in infants, the diagnosis of AOM will be made.
Pneumatic otoscopy, widely described in the international literature, is rarely used in our country, not even by specialists. Its possible added value would be in improving the sensitivity of visual otoscopy in the detection of SOM, approaching the results of objective techniques, such as impedanciometry. The technique is very simple, since it involves adapting a rubber insufflator to the air intake that many otoscopes have (Fig. 4).
Figure 4. Pneumatic otoscopy.
With the widest speculum that the child’s canal allows, negative pressure is applied (introduction of the fully collapsed insufflator into the auditory canal and subsequent dilation of the same, which should show approximation of the tympanic membrane towards the examiner), and then positive (contraction of the insufflator, which should show distance from the examiner’s tympanic membrane), observing the mobility of the tympanic membrane. The decrease or abolition of mobility to the application of negative pressure is suggestive of negative pressure in the middle ear. Decreased or abolished mobility upon application of positive pressure is suggestive of SOM. These changes are observed fundamentally in the postero-superior quadrant. As in conventional otoscopy, the duct must be clean of cerumen for an adequate assessment. The combination of the findings obtained with visual otoscopy and the assessment of tympanic mobility increases the sensitivity, specificity and positive and negative predictive values in the diagnosis of otitis media, but still does not present sufficient correlation with the results obtained with objective detection techniques of middle ear exudate. Pneumatic otoscopy is still a subjective assessment, thus experience is required to obtain adequate information, and an even greater degree of restraint of the child, since the duration and slight discomfort of the examination is somewhat greater, but the harmlessness and zero cost of the technique makes it highly recommended in PC consultations as an objective detection method of SOM.
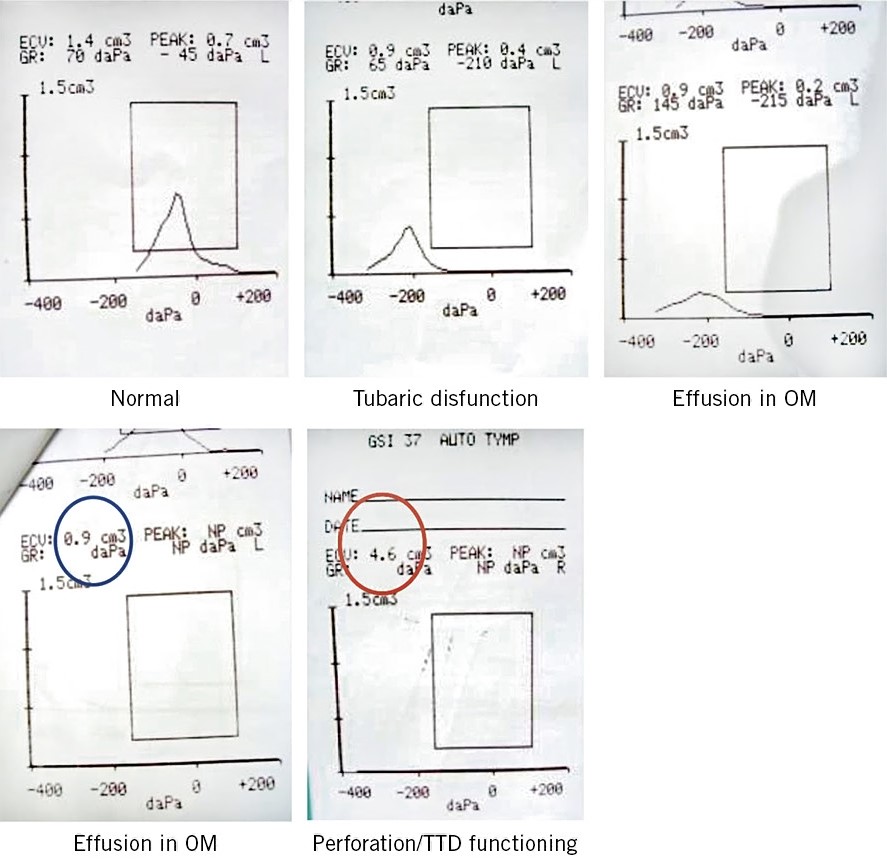
Due to the frequency of diagnosis of otitis media, the sometimes precarious conditions in which it occurs, and the repercussions on Public Health that failing to diagnose entails (generally due to excess), it is necessary for the PC pediatrician to have objective detection techniques for exudate in the middle ear. Tympanometry/impedanciometry is a validated non-invasive technique that makes it possible to predict the presence of said exudate with a sensitivity and specificity that approximates it to the gold standard in diagnosis, which is tympanocentesis, an invasive technique that should be reserved for highly selected situations. We must briefly describe tympanometry, since we consider it essential that the PC pediatrician becomes familiar with its use and interpretation in daily consultation(7). Tympanometry is based on the transfer of acoustic energy from an air medium with low resistance (impedance) to a liquid medium with high impedance (cochlea). If there is fluid in the middle ear that increases the resistance to the passage of sound, there will be a rebound of sound energy back towards the tympanic membrane, a situation that will be recorded by the device. The tympanometer automatically applies, in a single exploration, different pressures that modify the rigidity of the tympanic membrane. The changes can be seen in a curve called a tympanogram (Fig. 5), with 3 basic records: normal, with a pointed and centered curve; ETD (curves shifted to the left); and SOM (flat or undetectable curves).
Figure 5. Tympanogram curves. OM: otitis media. TTD: transtympanic drainage.
In addition, the tympanometer provides information about the pressures in the middle ear, which allows us to assess the presence of ETD (negative pressure in the middle ear, a frequent cause of otalgia, often associated with a common cold, not accompanied by middle ear exudate and, therefore, not amenable to treatment with ATB) and on the volume of the external auditory canal (EAC). When there is perforation, there is a continuity of cavities between the middle ear and the EAC, with a record of increased volume (>1 ml up to 7 years of age and greater than 2 ml in those over 7 years of age) and a diagnosis of tympanic perforation. This data is of extraordinary value for: detecting small perforations not otoscopically visible, assessing the functionality of the tympanic drains (children with functional drains must have volumes equivalent to a perforation) and making the differential diagnosis, which clinically is not always simple, between a suppurative otitis media (high volume due to perforation and continuity between the middle and external ear) and external otitis with otorrhea (normal volume, since there is no perforation or continuity). Tympanometry also allows us to differentiate between myringitis associated with a catarrhal process (hyperemia of the tympanic membrane, with intense otalgia, possibly with negative pressure in the middle ear or ETD, but without associated effusion) and AOM. A totally flat curve, a very common record in SOM, indicates middle ear effusion and negative pressure (ETD). Tympanometry offers reliable results from 7 months of age, since the distensibility of the external auditory canal in children under this age somewhat reduces its sensitivity, but its specificity is maintained. Modern tympanometers are very handy, lightweight, portable, not much larger than a conventional otoscope, which can even be transported in an emergency kit, and examination is fast, with the option of printing the record obtained, or viewing it and interpreting it live on the device screen. The graphs obtained are simple to assess, easily interpretable with minimal prior training, and the test is totally painless, being very well accepted by both children and their parents. Tympanometry shows the presence of middle ear effusion, but it does not allow us to differentiate between SOM and AOM, a differentiation that must be based on clinical interpretation.
Complications
With the exception of mastoiditis, the rest of the acute complications described in relation to AOM are currently exceptional. Hearing deficit is the most frequent complication in the medium and long term.
Serious complications in the acute phase (petrositis, labyrinthitis, facial paralysis, meningitis, brain abscess…) have been drastically reduced since the use of ATB in AOM. Only mastoiditis is observed with some frequency nowadays. Mastoiditis should be suspected whenever there is redness, swelling, and pain in the mastoid region. The suspicion should be confirmed by referring the child to the hospital for imaging studies. It has been speculated that the reduction in the use of ATB in AOM has been accompanied by a slight increase in the frequency of mastoiditis, but more recent studies(8) seem to rule out this hypothesis.
The most common complication is hearing loss, which is practically universal in the acute phase (25 dB average loss, equivalent to wearing earplugs) and is usually reversible once healing has been achieved. After an episode of AOM, 40% of patients present SOM at 4 weeks and 10% at 3 months, with variable functional repercussions that do not depend on the consistency of the fluid, so adequate control must be ensured after treatment confirming the disappearance of the effusion. The duration of the effusion does not seem to be related to the efficacy of the antibiotic treatment in its sterilization.
The PC pediatrician must monitor the possible appearance of complications in chronic otitis: the retraction of the tympanic membrane that is observed when the pressure in the middle ear is permanently negative, can be accompanied by cholesteatoma, a mass of stratified epithelium that invades the ossicular chain, with adhesion of the ossicles (adhesive otitis) or discontinuation/disarticulation between them, which generates potentially irreversible hearing loss. It may be suspected in the presence of a chronic perforation, with or without associated suppuration, or when a retrotympanic whitish mass is observed. In case of suspicion, the patient should be referred to a specialist.
It is more common to observe tympanosclerosis, white patches on the surface of the tympanic membrane, secondary to post-tympanic drainage scars or in children affected by recurrent AOM. Hearing should be assessed in these children, although it is generally a finding without functional repercussions.
Hospital referral
AOM is a pathology of exclusive initial management by the PC pediatrician. Referral should be made only when mastoiditis or neurological complications are suspected, and when exact knowledge of the causative germ is imperative or in situations of therapeutic failure in which tympanocentesis plays a diagnostic and therapeutic role in the resolution of otalgia.
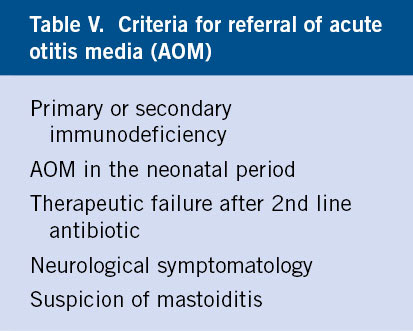
AOM is a disease managed practically exclusively by the PC pediatrician. Hospital referral will be made due to the need to perform tympanocentesis in the following situations (Table V):
• When it is a requisite to precisely identify the causative germ: primary or secondary immunocompromised and AOM in the neonatal period, frequently caused by gram negative and opportunistic germs.
• When there is symptom persistence (pain and/or fever) in spite of a correct standard antibiotic regimen (1st-line ATB, no improvement and change to 2nd-line ATB at 72 hours and new therapeutic failure). In this case, tympanocentesis has both diagnostic and therapeutic value, relieving persistent pain, and its performance should be considered an emergency. It is not reasonable to prescribe a third ATB, even if it is common practice, if the options handled previously were correct, with the possible exception of the use of ceftriaxone, which we will analyze later.
• When there are neurological symptoms: refer in case of suspicion of infrequent complications, such as: petrositis, meningitis, labyrinthitis and facial paralysis.
• When suspecting mastoiditis.
Treatment
The treatment of AOM must begin with correct analgesia. The use or not of ATB should be based on a correct diagnosis of AOM (with the presence of pain or its equivalent in infants) and on not treating positive otoscopy, but rather the combination of specific symptoms and suggestive otoscopic signs, preferably confirmed with pneumatic otoscopy and, if possible, with tympanometry. High-dose amoxicillin is the treatment of choice. Cefuroxime is the best option if a cephalosporin is used. Macrolides should not be used, except in case of anaphylactic allergy to penicillin. Ceftriaxone may be an option to consider if the parenteral route is required or as a last resort prior to hospital referral.
Analgesics
It is the first mandatory step (sometimes forgotten!) in managing AOM. The use of paracetamol at 15 mg/kg/dose every 4-6 hours (maximum 60 mg/kg/day in infants and 90 in those older than 1 year) is usually enough. Ibuprofen at 10 mg/kg/dose every 6 hours (maximum 60 mg/kg/day) is a reasonable option, especially if paracetamol is not accepted. There is less pediatric experience with magnesium metamizole, which should be reserved for cases of non-response to the above. The supposed advantage of using analgesics that have anti-inflammatory action motivates some pediatricians (and many parents) to prefer ibuprofen, but this advantage has not been conclusively demonstrated in any study. In case of therapeutic failure, it is useful to associate codeine (0.5-1 mg every 4 hours) with paracetamol, but never in children under 12 years of age. It is advisable, in the first 24 hours, to prescribe the analgesic at fixed doses, and later pro re nata (prn). The use of local heat can be useful. Topical analgesics should not be used, since they do not improve the results obtained with general analgesia, making subsequent otoscopy more difficult.
Antibiotics
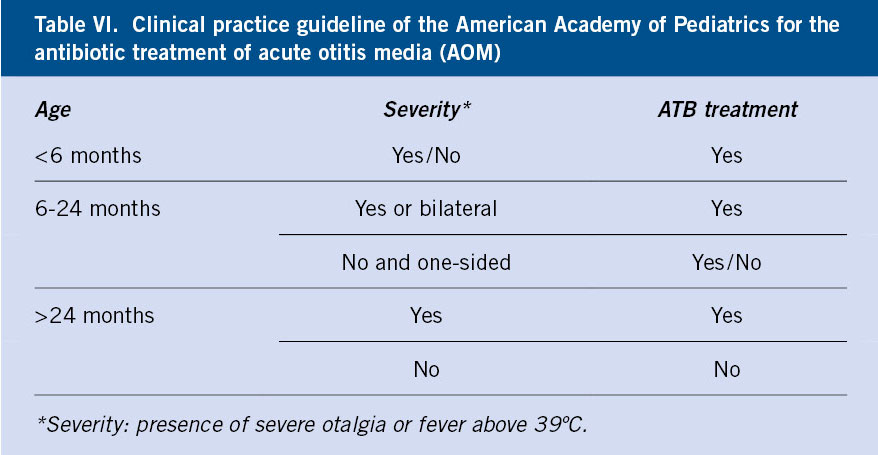
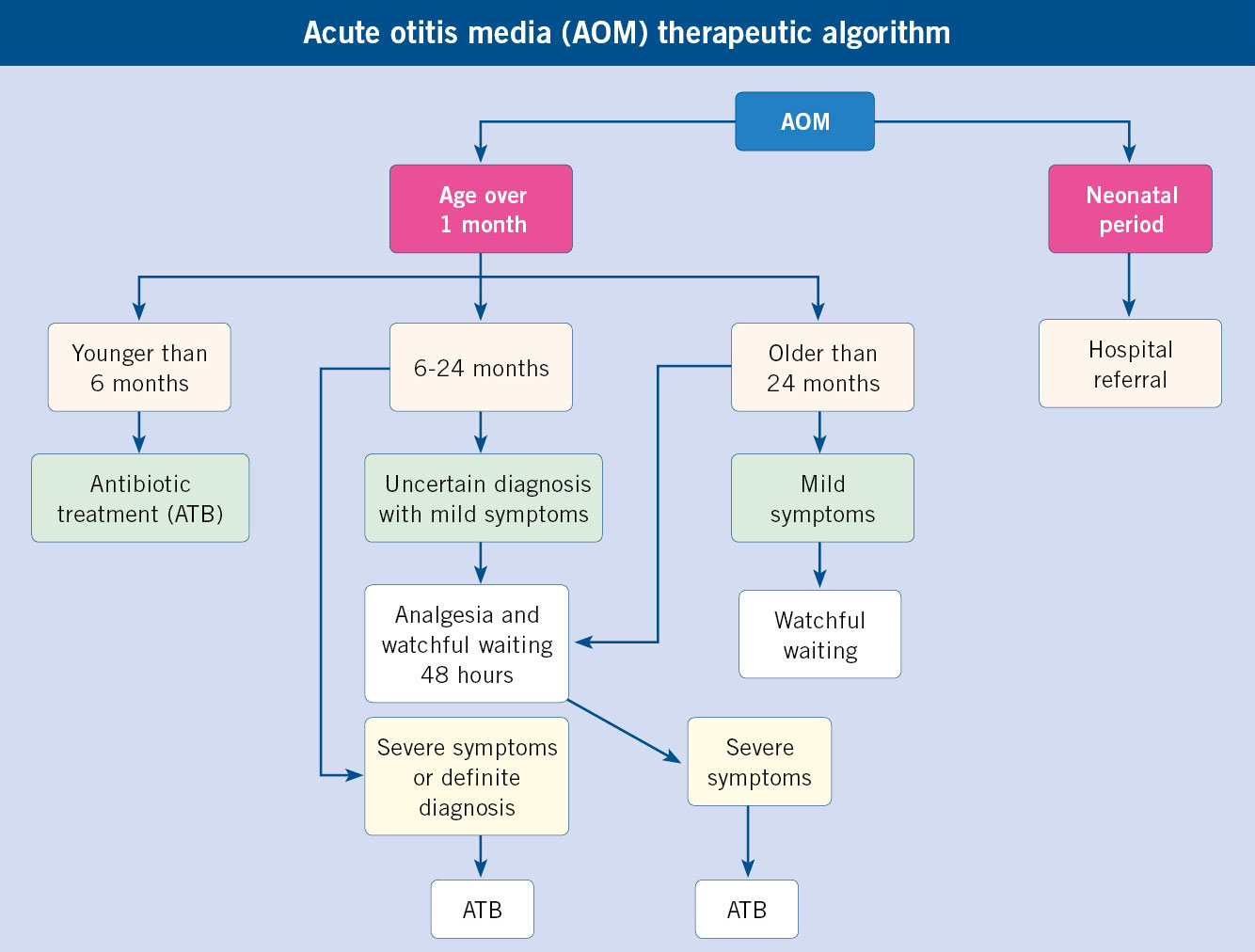
ATB treatment of AOM has always been a controversial topic. Several meta-analyses have agreed in concluding that, although most AOM resolve spontaneously, ATB treatment has a significant, albeit modest, effect on the improvement of acute symptoms (disappearance of fever and otalgia). According to different studies, between 8 and 17 cases of AOM would have to be treated with ATB to obtain symptomatic improvement in one (number of treatments needed to obtain improvement in 1 case or NNT). Recently, and with more restrictive diagnostic criteria, which tend to differentiate AOM from SOM more clearly, the NNT has been reduced to 3, enhancing the real value of ATB. Nowadays, It is considered that the main advantage of the use of ATB is in the prevention of short-term (mastoiditis) and long-term complications (chronic otitis media with conductive hearing loss and possible delay in language and school performance), despite the fact that this complication is currently being called into question. The so-called “Dutch guideline”, a name that is due to the fact that observational behavior began to be applied in the Netherlands, consisting of observation for 2-3 days without initial antibiotic treatment with a second evaluation to make a definitive decision, or in some cases, with the –in our opinion– inadvisable “deferred” prescription of ATB (“and if there is no improvement in 3 days, start administering it…”), which presupposes a capacity for evolutionary assessment and interpretation of symptoms by parents that is often not present, has initially been related to a slight increase in the number of mastoiditis in the countries where it is applied massively. The assessment of the real impact of this fact is being discussed, and does not seem to be confirmed in recent studies. Certainly, in the countries that treat AOM with the “Dutch guideline” (Holland, Scandinavia…) or “watchful waiting”, there is a much lower rate of resistance of pneumococcus to penicillin than in those where AOM is treated systematically. On the other hand, attention has been drawn to the conclusions of the meta-analyses that assess the effect of ATB on the evolution of AOM. In many of the analyzed works, there are no clear criteria for the AOM definition. It is very likely that many cases included in these works are actually SOM, in which the effect of ATB is much less or null, conditioning a marked similarity with the untreated control group. We believe that the objective of the PC pediatrician should be to treat authentic AOM and not positive otoscopy (differentiation is not always easy) and, above all, to avoid ATB in primarily viral respiratory pathology (common cold, most pharyngitis, laryngitis, acute bronchitis, bronchiolitis, pneumonitis). More studies are needed to definitively determine what factors are related to spontaneous resolution and absence of complications to leave AOM initially without ATB treatment. There are no conclusive data on this, however, based on current evidence, it seems reasonable to assume that in children over 2 years of age, with mild symptoms (mild ear pain and fever below 39ºC) and without general risk factors (chronic disease, immunodeficiency…) or local ones (tympanic perforation with suppuration, history of RAOM, surgical procedures related to middle ear pathology, such as tympanostomy, grommets, adenoidectomy) and unilateral AOM, the risk of not initially treating an AOM with antibiotics is minor, and can probably be safely assumed. In the clinical practice guideline of the American Academy of Pediatrics (AAP)(9), this minimum age for starting observational behavior is reduced to 6 months, considering this possibility as an alternative to ATB treatment between 6 and 24 months when the symptoms are mild and the AOM unilateral, and treating with ATB when the symptoms are severe (fever >39ºC or intense otalgia) or the AOM is bilateral (Table VI). It has been shown that the presence of bilateral AOM is more frequently related than unilateral AOM with: the presence of bacteria in the middle ear exudate, especially HI, greater tympanic inflammatory signs, younger age, and greater persistence of symptoms without ATB treatment. Studies that assess the follow-up of initial observation behaviors show that only 1/3 of cases resort to rescue ATB.
Once the need for ATB treatment has been established, its duration is also a matter of controversy. Classically, AOM have been treated for 10 days, by extrapolation from the treatment of streptococcal pharyngitis. However, streptococcus accounts for less than 5% of the etiology of AOM. There are studies that show similar clinical results with regimens of 3, 5, 7 and 10 days. On the other hand, there are more doubts about the effect of short treatments in the prevention of relapses and long-term sequelae. Given that these sequelae are more frequent in children under 2 years of age, who are underrepresented in studies of short regimens, the current recommendation is to treat these children, and those of any age with suppurative AOM (with perforation) for 10 days, and those of any age with suppurative AOM (with tympanic perforation), history of recurrent AOM, chronic otitis media or surgical procedures related to conductive hearing loss (adenoidectomy, transtympanic drainage, myringotomy) and reduce the duration to 5-7 days for older children without risk factors.
Current assessment of potentially usable ATB in AOM
We are going to refer only to the most used ones in our environment. The effectiveness of an ATB in the treatment of AOM depends fundamentally on the susceptibility of the pathogens causing it and on the ability of the drug to reach sufficient levels in the middle ear to eradicate the otopathogen. This ability is directly related to the time the ATB is above the minimum inhibitory concentration (MIC). This time is determined for beta-lactams at 40% of the dosage interval.
Amoxicillin; amoxicillin+clavulanic acid (A+C)
Amoxicillin, alone or in association with clavulanic acid, should be considered the treatment of first choice, due to its spectrum of activity, safety, tolerance and cost. Its minimum inhibitory concentrations (MIC) against pneumococcus are the lowest of all the oral ATB usable in Pediatrics and its penetration in the middle ear is only equaled by ceftriaxone. Clavulanic acid is a beta-lactam that, by itself, has little antibacterial activity and insignificant resistance-inducing capacity. Its function is the competitive, irreversible and suicidal inhibition of beta-lactamases, restoring amoxicillin to its initial spectrum, which it progressively lost, first against staphylococcus and, later, against HI and other beta-lactamase-producing germs. Following this concept, one must consider that, at present, we have two amoxicillins: one with a narrower spectrum, which at the AOM level has adequate activity against pneumococcus sensitive to penicillin (MIC < 2 ng/ml), and at high doses against pneumococcus with intermediate sensitivity (MIC 2-8 ng/ml) and even some strains of resistant pneumococcus (>8 ng/ml), and against Streptococcus pyogenes, but inactive against beta-lactamase-producing HI, Moraxella catarrhalis (100% beta-lactamase producer, but scarce presence in our environment) and Staphylococcus aureus. The other amoxicillin, associated with clavulanic acid, extends its activity to beta-lactamase-producing germs. The preference for one or the other will be given by the epidemiological situation. HI resistant to the A+C association have begun to be described, by a mechanism of alteration of penicillin-binding proteins similar to that of resistant pneumococcus, and not by production of beta-lactamases. The impact of this phenomenon for now seems insignificant. In our setting, there are a few studies on tympanocentesis and culture of middle ear exudate. The available data allow us to assume a 25% frequency of HI as the causative agent of AOM in the pre-vaccination era, but the increase in pneumococcal vaccination coverage seems to shift the etiological frequency towards the predominance of HI.
Some authors have defended the innocuousness and spontaneous resolution of AOM caused by non-typeable HI, as an argument to recommend treating only pneumococcus. However, there are studies that show the important role of HI in the production of relapses and chronic sequelae and, contrary to common belief, the clinical picture is not distinguishable from that caused by pneumococcus. Some authors propose the initial use of the A+C association in severe cases (fever above 39ºC and/or severe otalgia), but the greater intensity of the symptoms is not related to the HI etiology. Whichever amoxicillin is chosen, it should be used at doses not lower than 80 mg/kg/day, (maximum 3 g per day) divided into 2-3 doses and administered prior to food intake, which significantly improves tolerance. It is not necessary to use a strict schedule of every 8 hours. The division of the total dose in 3 shots at irregular intervals offers the same results. The use of high doses is especially indicated in children under 2 years of age attending school and in those who have recently received a beta-lactam. In other circumstances, it may be correct to start treatment with conventional doses (40-50 mg/kg/day). In current practice, high initial dosage has become the general norm, but there are no studies that quantify the therapeutic failure rate in both circumstances. The systematization of pneumococcal conjugate vaccines leads to a decrease in the prevalence of serotypes resistant to penicillin, therefore, dosage recommendations are likely to be revised downwards in the near future. In the US, presentations intended for administration in 2 doses have been approved, but there is little pediatric experience in this regard and doubts about its ability to eradicate pneumococcus in the middle ear, as conflicting data are presented on the time in which levels are maintained higher than the MIC in the interval between doses. The association with clavulanic acid in its current formulation of 8:1 facilitates reaching these doses without increasing gastrointestinal side effects, which are more frequently dependent on clavulanic acid than on amoxicillin. Clavulanic acid increases the peristalsis of the small intestine and more frequently produces diarrhea. Nevertheless, these effects are minimized with pre-meal administration, yogurt or probiotics, and rarely require discontinuation of treatment.
Cefuroxime axetil
It has the highest antipneumococcic activity of all the oral cephalosporins, but it is far inferior to amoxicillin in both potency and middle ear penetration. It is an option to consider in the frequent histories of doubtful allergies to penicillin with a history of non-anaphylactic symptoms in which the use of a beta-lactam is preferred to a macrolide, in those unknown or unusual patients in our consultation, cataloged previously, probably erroneously, as “allergic to penicillin”. It is an antibiotic of little acceptance by children due to its taste. It should be noted that cross allergies between penicillins and cephalosporins are much less frequent than previously thought, and are more frequent in first class cephalosporins (17%) than in second and third class cephalosporins (which are the ones that would be used in AOM, with 0.5%).
Cefixime
It has poor activity against pneumococcus with intermediate sensitivity or resistance to penicillin. On the other hand, it has the best activity against HI of all oral ATB, so it may be a good option, given its tolerability and dosage convenience, when its presence is strongly suspected, such as in homolateral AOM-purulent conjunctivitis syndrome.
Ceftriaxone
Its antipneumococcic potency and its excellent penetration into the middle ear make it a very interesting option for initial treatment with a single dose (50 mg/kg), an indication approved by the FDA, or in 3 successive doses every 24 hours when suspicion of penicillin-resistant pneumococcus, in situations of therapeutic failure, as the last step prior to referral for tympanocentesis. Given their high half-life, these 3 doses can also be administered every other day or even every 3 days, if necessary. In any case, its use must be individualized and strongly restricted to very special situations. It is an option to assess, especially in cases of marked intolerance to the oral route.
Clarithromycin
In terms of dosage convenience and tolerance, it is inferior to azithromycin.
Azithromycin
First-line treatment of choice for AOM in children with anaphylactic allergy to penicillin. However, it should not be the treatment of first choice in other circumstances, despite its convenient dosage, since it has been shown to be clearly inferior to amoxicillin in its ability to eradicate pneumococcus and haemophilus from the middle ear. The 3-day regimen and the 5-day regimen have been shown to have similar efficacy, so it seems more advisable to use the short regimen. The increasing use of macrolides in the 1990s led to a marked increase in resistant pneumococcus and Streptococcus pyogenes. A more restrained ATB policy in recent years has begun to reverse this trend. Contrary to pneumococcus resistance to beta-lactams, increasing the dose does not mean improving activity.
Clindamycin
30-40 mg/kg/day. It is an option to consider in children with anaphylactic allergy to penicillin who have experienced therapeutic failure with macrolides, but there is no pediatric presentation.
Progression control and therapeutic failure
The patient should be monitored if he does not improve after 72 hours, whether an antibiotic has been used or a watchful waiting has been decided. The slow resolution of otoscopic abnormalities makes it advisable to defer monitoring for several weeks after diagnosis.
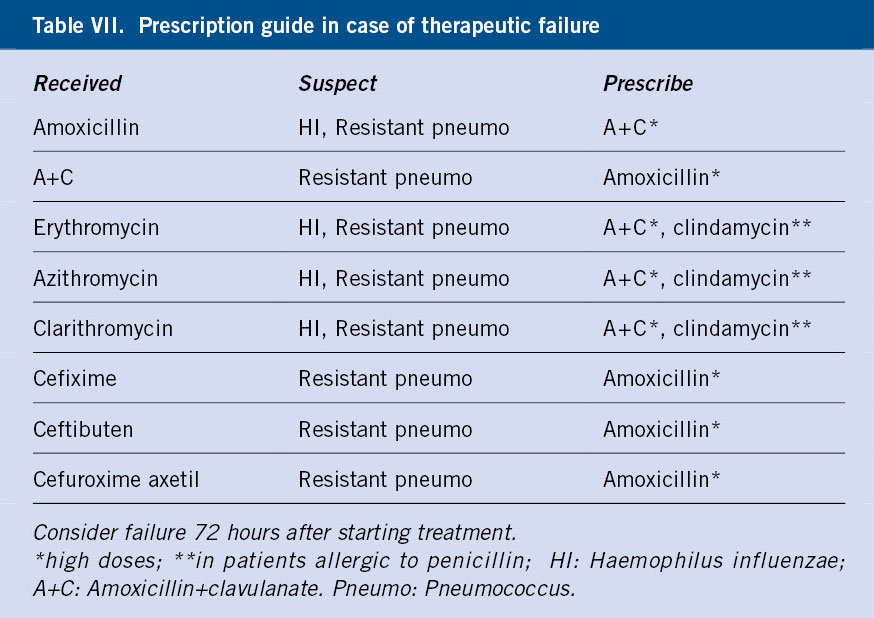
The growing incidence of resistant germs leads to an increase in therapeutic failures. This situation should be considered when, 72 hours after ATB treatment, the child continues to have pain and/or fever. This situation occurs in 15% of cases. In this case, a 2nd line ATB will be chosen according to the initial option and the suspicion of the germ involved (Table VII). If there is no response within 48 hours and always individualizing the case, the patient should be referred for tympanocentesis (which will relieve pain) and culture of the exudate. The difficulties of coordination with the specialized level that many pediatricians have in our environment means that, on many occasions, these children end up receiving a third ATB inappropriately, so at this point, the always very selective and restrictive use of ceftriaxone could be considered. The subsequent control of the disappearance of SOM should be done no earlier than 3-4 weeks after the end of treatment. In older children with mild sporadic AOM and without risk factors, it is even possible to consider not doing any control if the clinical evolution is favorable.
Acute otitis media with suppuration
Suppurative AOM should always be treated with ATB and receive the same therapeutic management as AOM with an intact tympanic membrane, except for the duration of antibiotic treatment, which should be 10 days.
Tympanic membrane perforation with evacuation of purulent exudate and eventually blood is so common in the evolution of AOM that it should be considered a form of clinical presentation rather than a complication. The pain usually goes away after the piercing. Therapeutic management should be done following the same action algorithm for AOM without suppuration, since the presence of suppuration has no implication regarding the suspicion of the germ involved, although in this case the isolation of Streptococcus pyogenes and staphylococcus increases. A very acute onset with intense otalgia and rapid suppuration was classically related as highly suggestive of pneumococcus, but this evolution is compatible with HI and other germs. ATB treatment should always last for 10 days, regardless of the child’s age. There is not enough documentation to support initial treatment with otic ciprofloxacin, which, on the other hand, is an excellent option in the event of therapeutic failure and in chronic suppurative otitis media (greater than two months), which were previously amenable to admission for parenteral antipseudomonal treatment. Mycobacterium tuberculosis is a rare cause of chronic suppurative OM. In chronic suppurative otitis media there is concomitant involvement of the mastoid cells and the presence of cholesteatoma must always be ruled out. Cholesteatoma is a benign tumor of the middle ear formed from epithelial remnants. It should be suspected when there is an opaque whitish coloration in the tympanic membrane, the presence of a polyp, caseous remains or persistent foul-smelling suppuration. You should always be referred to the ENT for surgery. A follow-up of the tympanic perforation should be done to verify its closure, and those that have not closed in 3 months should be referred to a specialist, for close follow-up and to assess the need for a plasty. Some recent studies seem to show superiority of the association of systemic antibiotic with topical ciprofloxacin in relation to the 2 options separately, but these data must be confirmed.
Recurrent acute otitis media (RAOM) and chronic otitis media with exudate (COME)
Definitions
RAOM is defined as: the presence of 3 or more episodes of AOM in 6 or fewer months, or 4 or more in 12 or fewer months, provided that the last episode occurred in the last 6 months. COME is defined as: the presence of bilateral exudate for more than 3 months or unilateral for more than 6 months. Frequently, both entities coexist.
A child is considered to suffer from recurrent acute otitis media when 3 or more episodes in a period equal to or less than 6 months or 4 or more in a period of less than 12 months present, provided that the last one has occurred in the last 6 months. This frequency has been significantly correlated with the appearance of medium- and long-term sequelae (COME) and/or conductive hearing loss. When otitis media with effusion, exudate or bilateral effusion persists for more than 3 months, or 6 months if it is unilateral, we speak of COME. Frequently, both pathologies coexist (RAOM with COME).
Epidemiology
Recurrent acute otitis media and chronic otitis media with exudate are the most frequent chronic problems in the PC pediatrician’s office.
80% of children under 3 years of age have had at least one episode of AOM, and 33% have had 3 or more attacks. RAOM is not usually the only manifestation of important immunological problems, and additional studies are not indicated in these children, unless there are other recurrent concomitant infections or other clinical signs that are suspicious for immunodeficiency. It is likely that certain mild and maturational immune problems may manifest only as RAOM. A significantly lower IgA level has been shown in these children, compared to children without RAOM. A relevant group of these children has significantly lower Ab responses to routine vaccinations than children without RAOM. The possible role of IgG2 subclass deficiency is discussed. In some studies, administration of gamma globulin to young children with RAOM showed some benefit. It has been reported that children with RAOM present non-protective responses against some common immunizations, benefiting from herd immunity in environments with high vaccination coverage(10). Regardless of the problems created by acute episodes, the primary objective of the PC pediatrician in managing this pathology must be to preserve hearing in the decisive phase of language learning. Numerous studies suggest that RAOM and COME in the first years of life have a significant adverse effect: on language comprehension, speech, reading and even the acquisition of gross motor functions, which is maintained up to 9 years of age. Follow-up studies up to 18 years of age, show a higher frequency of attention deficit hyperactivity disorder at 15 years, lower IQ at 13, and greater reading difficulties at 11-18 years in children with COME in the first years of life. In other studies, these results have been questioned, based on supposed methodological flaws. A multicenter study, with a strict control of the different and multiple variables, showed that there are no significant differences in language acquisition, learning or psychomotor or social development at 3 years, between children with COME who undergo early transtympanic drainage or late. The confusion of these data forces the pediatrician to handle them with caution and to assess the child with RAOM and/or COME as a child at risk for a possible hearing-learning problem, perhaps being more conservative in the treatment than in general practice.
Recurrent acute otitis media
Certain factors favor its presence (Table VIII): male sex, sibling with a history of RAOM, first attack of AOM before 4 months of age, primary or secondary immunodeficiency, artificial feeding, attendance to a nursery with more than 6 children in the group, passive smoking, pacifier use beyond 6 months, presence of apparent or hidden cleft palate (uvula bifida), Down syndrome and sensitization to allergens.
Actions
Before addressing a possible case of RAOM, the PC pediatrician should be very careful in labeling a patient in this way, since the diagnosis is rarely established based on correct documentation of the different episodes of AOM, actually confusing SOM with AOM. Diagnoses made without objective detection methods of exudate in the middle ear are unreliable without the presence of very specific symptoms or otoscopy (ear pain, bulging eardrum). On many occasions, any catarrhal or febrile condition without focality in which the otoscopy suggests SOM is classified as AOM.
The initial step is to eliminate predisposing factors whenever possible: suggest to parents the possibility of withdrawing, at least temporarily, from the nursery, avoiding passive smoking, exposure to allergens, vaccinating against influenza in the indicated period (with the reduction of up to 40% of AOM episodes in the epidemic period in children who go to nursery school in some studies, and without significant differences in others) and preventive restriction of the use of pacifiers in children older than 6 months at the time of sleep conciliation, with absolute withdrawal from 10 months (reduction of up to 33% of AOM attacks in some studies). The 13-valent pneumococcal conjugate vaccine has shown a significant reduction in the number of episodes. Some studies showed that xylitol chewing gums have a significant beneficial effect, but at unrealistic doses (5 chewing gums a day, since 3 are not effective, and during the entire epidemic period of respiratory infections). The PC pediatrician must also rule out pathology associated with ROAM, such as: immunodeficiencies, which will only manifest with ROAM without other pathologies in exceptional cases; primary ciliary dyskinesia; and the (rare) cavum neoplasm. However, it is likely that the child with AOM presents alterations in the immune response, both innate and adaptive, to the otopathogens that cause AOM. It has been shown that the presence of a varied flora in the nasopharynx, specifically that of Corynebacterium spp and Dolosigranulum, is a protective factor for the development of ROAM(11).
Although abundant literature showed that ROAM could be adequately managed in PC with antibiotic prophylaxis for a period of time from 3 (spring-summer) to 6 months (autumn-winter), which significantly reduced the number of episodes (1.04/child/year with placebo to 0.28/child/year with ATB), this practice has been abandoned due to the growing problem of inducing antibiotic resistance. When clinical symptoms of persistent nasal obstruction are present, almost certainly due to hypertrophy of the adenoids, specialized entry referral for adenoidectomy is reasonable. Adenoidectomy associated with drains is also recommended in a hypothetical second surgical treatment in children, who after the first expulsion of the same maintain their pathology(12), regardless of its size, due to the benefit of eliminating a focus of chronic infection associated with the generation of biofilms and the inflammation of adenoids that alters the function of the Eustachian tube. In the absence of obstructive symptoms, referral or not to a specialist will be individualized based on the time of year (abstention is recommended in the summer), the presence or absence of COME and/or symptoms compatible with conductive hearing loss, and of the possibility or not that the PC pediatrician has objectively documented in his consultation the presence of exudate in the middle ear (impendancemetry) and its functional repercussion (otoacoustic emissions, audiometry in older children). It has been reported that there is a relationship between RAOM and low levels (<30 ng/ml) of vitamin D and it has been suggested(13) that supplementation with 1000 IU/day of vitamin D for 4 months significantly reduces the number of episodes. More studies are needed to make a recommendation in this regard. Sleeping in the prone position is associated with an increased risk of RAOM.
Chronic otitis media with exudate
Chronic otitis media with exudate is defined as: the presence of otitis media with bilateral effusion lasting more than 3 months or with unilateral effusion lasting more than 6 months.
Although the fluid may be transudate or exudate, children with SOM or COME may experience exacerbations (AOM) and the culture obtained by tympanocentesis shows, in a high percentage of cases, the growth of the same germs that cause AOM. COME is always associated with conductive hearing loss with variable functional repercussions and, on some occasions, with reversible sensory hearing loss (due to pressure and rigidity on the round window membrane) or permanent (due to the spread of the infection or the products of inflammation through the round window membrane, by the development of a perilymphatic fistula in the round window or by suppurative labyrinthitis). Visual otoscopy is not sensitive enough to detect many cases of SOM and, consequently, of COME. Otoscopic signs suggestive of SOM are: hyperemia associated with dullness, yellowish coloration and bluish coloration of the tympanic membrane. The latter is especially associated with COME. The visualization of a retraction in the tympanic membrane, in which the ossicles of the middle ear will be very marked, is highly suspicious of negative pressure in the middle ear (ETD) and correlates with COME. Although pneumatic otoscopy improves the sensitivity of visual otoscopy, it requires experience to interpret the results and is also a subjective interpretation examination. In a COME we will observe immobility of the tympanic membrane, both in the application of negative and positive pressure. Only impendatiometry has a sensitivity and specificity close to tympanocentesis. Unfortunately, the PC pediatrician does not usually have this technique.
We can reach the diagnosis of COME via 3 ways (Table IX):
• AOM follow-up in which the SOM does not resolve within 3 months (bilateral) or 6 months (unilateral).
• SOM detected in a routine health check, whose follow-up in 3 months (bilateral) or 6 months (bilateral) leads to the diagnosis of COME (called “de novo”).
• COME diagnosed in a child who consulted for long-standing hearing loss or compatible associated symptoms (language delay, school delay).
The diagnosis of COME, whether suspected by visual or pneumatic otoscopy, or preferably by confirmation with objective techniques for detecting middle ear exudate (impedanciometry), should require a specialized referral to assess the functional repercussion, since the transtympanic drainage associated or not to adenoidectomy, depending on the presence or not of associated nasal obstruction symptoms, is the treatment of choice for COME with conductive hearing loss greater than 30 dB. In our environment, the collaborative relationships between both levels and the saturation of reference specialists makes it difficult for this referral to be productive, with which the PC pediatrician retains many cases in which the manifestations seem to be of less relevance or there is no apparent hearing impairment or alteration in school performance. The objective assessment of the functional repercussion of COME is not usually available for many PC pediatricians until 6 years of age, the age at which a normal child collaborates in a conventional audiometry. However, the impact of this pathology occurs at much lower ages. The possibility that the PC pediatrician could have an otoacoustic emissions detector in his consultation, which allows ruling out significant conductive or sensory hearing loss, with a cut-off level of 30 dB, would be a very significant advance in the outpatient management of this pathology(14).
The intervention is well tolerated and during the time the drains remain in the middle ear (6-18 months), the child’s hearing significantly improves and exacerbations decrease. Specialists do not usually follow up these children post-intervention, but a hearing assessment is required after it. A small percentage has persistent otorrhea, which responds well to topical ciprofloxacin. In clinical practice guidelines, it is recommended that the initial approach to COME should be more conservative and that referral for surgery seems especially indicated (Table X) in cases of: cleft palate, retraction or atelectasis of the tympanic membrane, suspected cholesteatoma, bilateral involvement, suspected delay in language or psychomotor development in general, autism spectrum disorders or congenital syndromes associated with developmental or language delay(15). Several studies have been published in which medical treatment is used before proceeding with surgery. Good results were obtained with the use of antibiotics for a month at therapeutic doses, associated with oral corticosteroids in the last week. However, the benefits obtained were short-term and, in any case, the aggressiveness of this regimen entails the need to individualize the case before using it very punctually. However, a therapeutic attempt with a course of antibiotics for 2 weeks, if they have not been used recently, seems a reasonable attempt before surgery and is commonly used by many pediatricians. In any case, given the high rate of spontaneous resolution of SOM in summer, it is common practice to let this season pass before any surgical approach.
The use in COME of therapies with nasal decongestants, mucolytics or antihistamines, alone or in association, is frequent. No controlled study has shown any benefit from their use. It is likely that antihistamines have some beneficial effect on SOM in patients with associated allergy, but this has not been studied.
Otorrhea in children with transtympanic drainage (TTD)
It is a frequent complication that must be treated with topical ciprofloxacin.
Otorrhea occurs in 20-50% of children with drains. Acute otorrhea should be considered as another episode of AOM. In contrast, the absence of otorrhea in a carrier of functional DTT rules out the possibility of AOM. The responsible germs are the same as in unoperated children, and the flora that comes from the external auditory canal must also be considered (Pseudomonas aeruginosa, Staphylococcus aureus and Proteus mirabilis). There is a growing isolation of community-acquired methicillin-resistant staphylococci (MRSA). The use of topical ciprofloxacin is the first choice, as it has been shown to be as effective as amoxicillin/clavulanic acid and superior in bacteriological eradication, with few local side effects and without induction of resistance. In recurrent or persistent cases, the combination of both treatments, topical and systemic, has been successfully tested. Recent studies suggest that the association of topical use of ciprofloxacin with corticosteroids is superior to ciprofloxacin alone. The failure of topical treatment makes it advisable to perform a culture, since quinolones slightly increase the risk of fungal superinfection.
External otitis
External otitis is generally caused by excess moisture in the ear canal. Hygiene standards and local antibiotic treatment will suffice in most cases.
External otitis is the inflammation and/or infection of the external auditory canal. In the daily practice of the PC pediatrician it is important to recognize: diffuse external acute otitis, circumscribed external acute otitis or furuncle of the ear canal and bullous or bullous myringitis.
Diffuse acute external otitis (“swimming pool otitis”)
Persistent moisture in the ear canal: alters local immune mechanisms, decreases the antimicrobial activity of cerumen, the thickness of the keratin layer of the epithelium, increases the pH, macerates the skin and microcracks appear. However, extreme dryness, the complete absence of earwax (which acts against moisture) or an impacted earwax plug (which retains water) are also predisposing factors for external otitis. The etiology can be bacterial, generally due to Pseudomonas (80%), which overgrows in a humid environment, and staphylococcus (epidermidis and aureus), but also due to enterobacteria (Proteus mirabilis), diphtheroids or citrobacter, mycotic (candida in immunodeficient patients and Aspergillus in diabetics) or viral: herpes simplex and varicella-zoster (Ramsay-Hunt syndrome). Diffuse otitis externa also appears as a frequent complication of otorrhea in carriers of transtympanic drainage(16).
Clinical manifestations
These are fundamentally characterized by pain, sometimes preceded by itching, with the classic tragus and pinna signs, which are very positive. It is normally one-sided. Usually, there is clinical-otoscopic dissociation, since the child has much greater pain than the few or no inflammatory signs that can be seen in the initial phases. Severe pain is due to innervation of the external auditory canal by 4 cranial nerves. Subsequently, hyperemia of variable intensity appears in the skin of the external auditory canal and narrowing of up to 50% of its lumen, with difficulties in visualizing the tympanic membrane. Hearing loss is common and suppuration is not uncommon. Fever is not frequently observed and, when it occurs, it usually indicates a mixed staphylococcal-Pseudomonas infection. It may be accompanied by preauricular adenopathy.
The differential diagnosis with AOM is not always easy, especially when there is suppuration that prevents visualization of the tympanic membrane. The pain usually improves when there is perforation in the AOM and, instead, worsens with otorrhea in external otitis. In case of doubt, the tympanometer is an essential ally, verifying whether or not there is tympanic perforation with the assessment of the volume of the external ear. The tragus and pinna signs are typical of external otitis and not of AOM. In AOM there is usually a history of catarrh, and fever is more common. External otitis is more typical of summer. If external otitis presents with periauricular edema, it must be differentiated from mastoiditis, in which there is usually a previous episode of AOM, hearing loss, pain on pressure of the mastoid process, and the absence of the tragus sign.
Treatment
The main treatment is the analgesic, since pain is the capital sign. The use of paracetamol or ibuprofen at adequate doses is usually sufficient. Local heat is helpful. In more severe cases, a good response is obtained with the association of paracetamol and codeine, which should not be used in children under 12 years of age. If there is a lot of organic material and exudate, the ear canal should be cleaned with a curette or irrigation, and if it is not possible to do so in the office, the patient should be referred to the ENT specialist. If this cleaning is not done, the topical ATB will not contact the canal wall and the treatment will fail. If the duct is patent, local ATB should be administered. Classically, preparations have been used that associate aminoglycosides (neomycin, polymyxin or colistin) with corticosteroids, at the rate of 2 drops, 4 times a day for 7 days. There are no studies that compare the additional advantage of the use of corticosteroids with respect to the use of ATB alone. For the use of these classic preparations, it must be certain that the tympanic membrane is not perforated, which can be difficult if there is secretion, if a tympanometer is not available, since ototoxicity has been described on rare occasions. These preparations contain propylene glycol and acetic acid, which are irritating and can cause contact eczema. On the other hand, sensitization reactions to topical aminoglycosides have been described. Otic ciprofloxacin is equally effective and, despite the fact that its cost is much higher, it seems to be the most recommended option at present, due to its better tolerance, more comfortable dosage (twice a day) and absence of side effects and sensitization. The child with the usual mild external otitis can continue to bathe, as long as he does not dive in the course of treatment.
Despite its theoretical banality, external otitis is not exempt from potential complications, so the PC pediatrician should refer all external otitis with fever, which will require culture of the secretion and probable parenteral treatment, and whenever no improvement is obtained with 7 days of local treatment.
The prevention of recurrences is essential in children who practice swimming. They must be educated to carefully dry the canal after coming out of the water, using the corner of the towel, since humidity is the main breeding ground for Pseudomonas overgrowth. It can be very useful in recurrent cases, the instillation of a few drops of acetic acid (common kitchen vinegar) diluted 50% in water, in the external auditory canal when leaving the pool, since the reduction of the pH in the auditory canal prevents bacterial proliferation, especially that of Pseudomonas. Electric dryers for external auditory canal have recently been marketed, but a conventional hair dryer is also helpful. The usefulness of the use of plugs is controversial.
Circumscribed acute external otitis (furuncle of the external auditory canal)
Caused by Staphylococcus aureus, it should receive initial systemic treatment with cloxacillin or ATB with anti-staphylococcal activity, associated with adequate analgesia. If the condition is severe or does not respond to treatment in 3 days, the child should be referred to the ENT to proceed with drainage.
Myringitis
Myringitis is inflammation of the tympanic membrane. It is common to observe tympanic hyperemia with otalgia in the course of upper tract viral symptoms, without the presence of middle ear exudate. Once again, the tympanometer will offer us the differential diagnosis between simple myringitis and AOM. These catarrhal forms should not receive antibiotic treatment, although their evolution should be monitored, since they can evolve into AOM. The visualization of tympanic bullae (which must be differentiated from the presence of retrotympanic liquid levels, typical of chronic forms of otitis media with exudate) associated with intense pain, is diagnostic of bullous or bullous myringitis. Bullous myringitis is a form of otitis media involving the tympanic membrane, which, although previously considered to be due to Mycoplasma pneumoniae, has been shown that it is most frequently caused by the usual AOM-producing germs (pneumococcus, Haemophilus…). The initial treatment will be identical to that of AOM. Therapeutic failure should make us consider the possibility of establishing a macrolide with activity against Mycoplasma. Mycoplasma myringitis rarely occurs without concomitant involvement of the lower respiratory tract (tracheobronchitis, pneumonia).
Necrotizing external otitis (“malignant external otitis”)
It is the infection of the cartilage and bone of the external auditory canal. It is a rare entity that should be known by the PC pediatrician given its potentially severe nature. It affects infants, diabetics and immunocompromised. It is usually caused by Pseudomonas and is characterized by: fever, poor general condition, toxicity, necrosis of the auditory canal, extension to the middle and inner ear, facial paralysis and CNS involvement. It can be diagnosed in the initial phase, with the visualization of granulation tissue in the cartilage-bone transition zone of the external auditory canal. Upon suspicion, the child should be urgently referred to the hospital for surgical treatment. Without treatment, mortality rises to 80%.
Conflict of interests
There is no conflict of interest in the preparation of the manuscript. Declaration of interests: none.
Bibliography
Asterisks reflect the interest of the article in the author’s opinion.
1.** Pichichero ME. Otitis media. Pediatr Clin N Am. 2013; 60: 391-407.
2.* Kaur R, Casey JR, Pichichero ME. Relationship with original pathogen in recurrence of acute otitis media after completion of amoxicillin/clavulanate. Pediatr Infect Dis J. 2013; 32: 1159-62.
3.* Pichichero ME. Ten-year study of acute otitis media in Rochester, NY. Pediatr Infect Dis J. 2016; 35: 1027-32.
4.*** Pumarola F, Mar ès J, Losada I, Minguella I, Moraga F, Tarragó D, Ulla G, et al. Microbiology of bacteria causing recurrent acute otitis media (AOM) and AOM treatment failure in young children in Spain: Shifting pathogens in the post-pneumococcal conjugate vaccination era. International Journal of Pediatric Otorhinolaryngology. 2013; 77: 1231-6.
5.* Nokso-Koivisto J, Marom T, Chonmaitree T. Importance of viruses in acute otitis media. Curr Opin pediatr. 2015; 27: 110-5.
6.** Chonmaitree T, Ruohola A, Hendley JO. Presence of viral nucleic acids in the middle ear. Acute otitis media pathogen or bystander? Pediatr Infect Dis J. 2012; 31: 325-30.
7.* De la Flor J, Parellada N. Correlació d’otoscòpia visual, otoscòpia pneumàtica i timpanometria en la detecció de disfunció de trompa d’Eustaqui i d’exudat d’oida mitjana. Pediatr Catalana. 2003; 63: 62-8.
8.** Anthonsen K, Hostmark K, Hansen S, Andreasen K, Juhlin J, Homoe P, et al. Acute mastoiditis in children: a 10 year retrospective and validated multicenter study. Pediatr Infect Dis J. 2013; 32: 436-40.
9.*** Leiberthal AS, Carroll AE, Chonmaitree T. Clinical practice guideline: the diagnoses and management of acute otitis media. Pediatrics. 2013; 131: e964-9.
10.* Pichichero ME, Casey JR, Almudevar A. Nonprotective responses to pediatric vaccines occur in children who are otitis-prone. Pediatr Infect Dis J. 2103; 32: 1163-8.
11.* Folino F, Fattizzo M, Roggiero L, Oriano M, Aliberti S, Blasi F, et al. Nasopharyngeal microbiota analysis in healthy and otitis-prone children. Pediatric Infect Dis J. 2021; 40: 16-21.
12.* Kujala T, Alho OP, Luotonen J, Kristo A, Uhari M, Renko M, et al. Tympanostomy with and without adenoidectomy for the prevention of recurrences of acute otitis media. Pediatr Infect Dis J. 2012; 31: 565-9.
13.* Marchisio P, Consonni D, Baggi E, Zampiero A, Bianchini S, Terranova L, et al. Vitamin D supplementation reduces the risk of acute otitis media in otitis-prone children. Pediatr Infect Dis J. 2013; 32: 1055-60.
14.* Balatsouras DG, Kokoutsis G, Ganellis P. Transiently evoked otoacoustic emissions in children with otitis media with effusion. Int J Otolaryngol. 2012; 2012: 269203.
15.** Rosenfeld RM, Schwartz SR, Pynnonen MA. Clinical practice guideline: tympanostomy tubes in children. Otolaryngol Head Neck Surgery. 2013; 149: S1-S35.
16.** Rosenfeld RM, Schwartz SR, Cannon CR. Clinical practice guideline: acute otitis externa. Otolaryngol Head Neck Surg. 2014; 150: S1-S24.
Recommended bibliography
– American Academy of pediatrics. Swimmer’s ear/acute otitis externa. In: Kimberlin DW, Brady MT, Jackson MA, Long SS. Red Book: 2015 Report on the Committee on infectious diseases. 30th ed. Elk Grove Village IL: American Academy of Pediatrics; 2015. p. 218.
– Arnold DH, Spiro DM. Otitis media with effusion. In: Bajaj L, Hambidge SJ, Kerby G, Nyquist AC. Berman’s pediatric decision making. 5th ed. Elsevier. Philadelphia; 2011. p. 128-31.
Updated guide in a pediatric algorithms text.
– Boyce TG, Balakrishnan K. External otitis and necrotizing external otitis. In: Long SS, Prober CHG, Fischer M. Principles and practice of pediatric infectious diseases. 5th ed. Elsevier. Phidalephia; 2017. p. 223-5.
– Casey JR, Bluestone CHD. Middle ear. In: Cherry JD, Harrison JG, Kaplan SL, Steinback WJ, Hotez PJ. Textbook of pediatric infectious diseases. Saunders Elsevier. 8th ed. Philadelphia; 2018. p. 14969.
– Friedman EM, Carrillo M. External otitis. In: Cherry JD, Harrison JG, Kaplan SL, Steinback WJ, Hotez PJ. Textbook of pediatric infectious diseases. Saunders Elsevier. 8th ed. Philadelphia; 2018. p. 1449.
– HaddadJ, Dodhia SN. External otitis (external otitis). In: Kliegman RM, St. Geme III JW. Nelson textbook of pediatrics. 21st edition Elsevier. Philadelphia; 2020. p. 3414-7.
– Hullegie S, Venekamp R, van Dongen T, Hay AD, Moore MV, Little P, et al. Prevalence and antimicrobial resistance of bacteria in children with acute otitis media and ear discharge. Pediatr Infect Dis J. 2021; 40: 756-62.
– Kerschner JE, Preciado D. Otitis media. In: Kliegman RM, St. Geme III JW. Nelson textbook of pediatrics. 21st edition Elsevier. Philadelphia; 2020. p. 3418-31.
Complete and updated review in the most consulted general Pediatrics textbook in the world. 15 must-read pages for the PC pediatrician.
– Pelton YES. Middle ear. In: Long SS, Prober CG, Fischer M. Principles and practice of pediatric infectious diseases. 5th ed. Philadelphia: Elsevier; 2017. p. 216-23.
The fundamentals in otitis media, condensed in 7 essential pages of a treatise on pediatric infectious diseases. Ideal for PC pediatricians with little time for reading.
– Pichichero ME. Ten-year study of the stringently defined otitis-prone child in Rochester, NY. Pediatr Infect Dis J. 2016; 35: 1033-9.
– Spire DM, Arnold DH. Acute otitis media. In: Bajaj L, Hambidge SJ, Kerby G, Nyquist AC. Berman’s pediatric decision making. 5th ed. Elsevier. Philadelphia; 2011. p. 124-7.
Guide in a text of pediatric algorithms.
– Schwartz SR, Coggins R, Gagnon L, Hackell JM, Hoelting D, Hunter LL, et al. Clinical Practice Guideline: Otitis Media with Effusion Executive Summary (Update). Otolaryngology–Head and Neck Surgery. 2016; 154: 201-14.
– Wald ER, De Muri, GP. Antibiotic recommendations for acute otitis media and acute bacterial sinusitis. Conundrum no more. Pediatr Infect Dis J. 2018; 37: 1255-7.
– Pichichero ME. Middle ear. Pediatr Clin N Am. 2013; 60: 391-407.
Brief review of the most important concepts of the disease.
– Chonmaitree T, Ruohola A, Hendley JO. Presence of viral nucleic acids in the middle ear. Acute otitis media pathogen or bystander? Pediatr Infect Dis J. 2012; 31: 325-30.
Interesting controversial article on the real role of viruses in the etiology of AOM.
– De la Flor J, Parellada N. Correlació d’otoscòpia visual, otoscòpia pneumàtica i timpanometria en la detecció de disfunció de trompa d’Eustaqui i d’exudat d’oida mitjana. Pediatr Catalana. 2003; 63: 62-8.
Work carried out in the field of PC with a large sample of patients, in which the need to objectively detect middle ear exudate as much as possible is demonstrated.
– Kujala T, Alho OP, Luotonen J, Kristo A, Uhari M, Renko M, et al. Tympanostomy with and without adenoidectomy for the prevention of recurrences of acute otitis media. Pediatr Infect Dis J. 2012; 31: 565-9.
Excellent work in which it is shown that the first surgical approach of the child with COME/RAOM should be based only on transtympanic drainage, except if there is are relevant obstructive nasal signs.
| Clinical case 1 |
|
A 3-year-old boy, with no background history of interest, attends the consultation with a clinical picture of a few hours of right otalgia, in the course of a catarrhal process that began 5 days ago. The mother has administered a dose of paracetamol, which has eliminated the pain. On physical examination, we observe an afebrile child, in good general condition, hyperemic pharynx, clear mucus in the cavum and watery rhinorrhea. The tragus sign is negative. Pneumatic otoscopy shows us an intense hyperemia of the left tympanic membrane, without the presence of bulging or exudate in the external auditory canal. The mobility of both membranes under positive and negative pressure is abolished. Tympanometry shows a completely flat left curve.
|
| Clinical case 2 |
|
A 5-year-old boy with no history of interest comes to the clinic with a clinical picture of fever lasting 3 days, accompanied by a long-standing catarrhal picture and intense otalgia. Physical examination shows a congested pharynx, thick mucus in cavum, rhinitis, otoscopy with marked hyperemia and yellowish discoloration of the tympanic membrane. With the diagnosis of AOM, treatment with amoxicillin was started at a dose of 80 mg/kg/day. On the third day, the child comes back as he has not experienced any improvement, despite adequate compliance and oral route acceptance, and thus, a 2nd line antibiotic was prescribed. 48 hours later the child remains febrile and continues to have otalgia.
|
| Clinical case 3 |
|
This is a 2 and a half year old boy, attending a nursery school. He is a regular patient in our practice, with frequent catarrhal processes and a history of 7 well-documented AOM episodes in a period of 11 months. His last acute process was 3 months ago. Coinciding with the summer period, expectant management was decided. The boy has received several rounds of antibiotic treatment in recent months, often on weekends in emergency services. The current reason for consultation is that the mother is concerned about the child’s language. He is not able to put two words together and his vocabulary is very restricted. At the 18-month and 2-year check-ups, the M-CHAT autism screening questionnaire was normal. They attach a school report describing non-participatory behavior. The physical examination does not show significant findings, except for the presence of an otoscopy with bluish eardrums, slight tympanic retraction, pneumatic otoscopy with total immobility of both tympanic membranes and a completely flat tympanogram. These same findings are documented multiple times in history since the last AOM episode.
|
 Upper respiratory tract infections-2: otitis media (etiology, symptoms and diagnosis; complications and treatment); recurrent acute otitis media and chronic otitis media; external otitis
Upper respiratory tract infections-2: otitis media (etiology, symptoms and diagnosis; complications and treatment); recurrent acute otitis media and chronic otitis media; external otitis