|
| Topics on Continuous Training |
R. Bazire Batiz*, C. Morales Cabeza**
*Allergy Service. Niño Jesús University Children’s Hospital. Madrid. **Allergy Service. Puerta de Hierro University Hospital. Majadahonda. Madrid
| Abstract
The excess of unconfirmed drug hypersensitivity diagnoses is a significant clinical problem because of patient therapeutic compromise. Primary care physicians are often the first point of contact for children with drug adverse reactions, so it is important that they are able to assess and manage these patients and refer those who require an allergy study. |
| Resumen
El exceso de diagnóstico no confirmado de hipersensibilidad a fármacos constituye un problema relevante en la práctica clínica, por la limitación terapéutica que implica para los pacientes. Los médicos de Atención Primaria son, a menudo, el primer punto de contacto para los niños que sufren reacciones, por lo que es importante saber evaluarlas, tratarlas, y derivar a los pacientes que requieran un estudio alergológico. |
Key words: Adverse drug reactions; Drug allergy; Children.
Palabras clave: Reacciones adversas a fármaco; Hipersensibilidad a fármaco; Niño.
Pediatr Integral 2023; XXVII (3): 162-172
• To learn how to distinguish a hypersensitivity reaction from other adverse drug reactions.
• To recognize the key points of the clinical history of a hypersensitivity reaction to drugs.
• To identify the severe signs and symptoms of a drug hypersensitivity reaction.
• To familiarize with the recommendations that should be provided to a patient with suspected hypersensitivity reaction.
• To determine when to refer a patient to specialized care so as to carry out allergological studies.
Allergy to medicines and drugs
Introduction
Type A adverse drug reactions (ADRs) are those reactions that are foreseeable and related to the known pharmacological effects of the drug itself. Type B ADRs are less common and are due to an abnormal response in predisposed individuals. Hypersensitivity reactions are included in type B reactions.
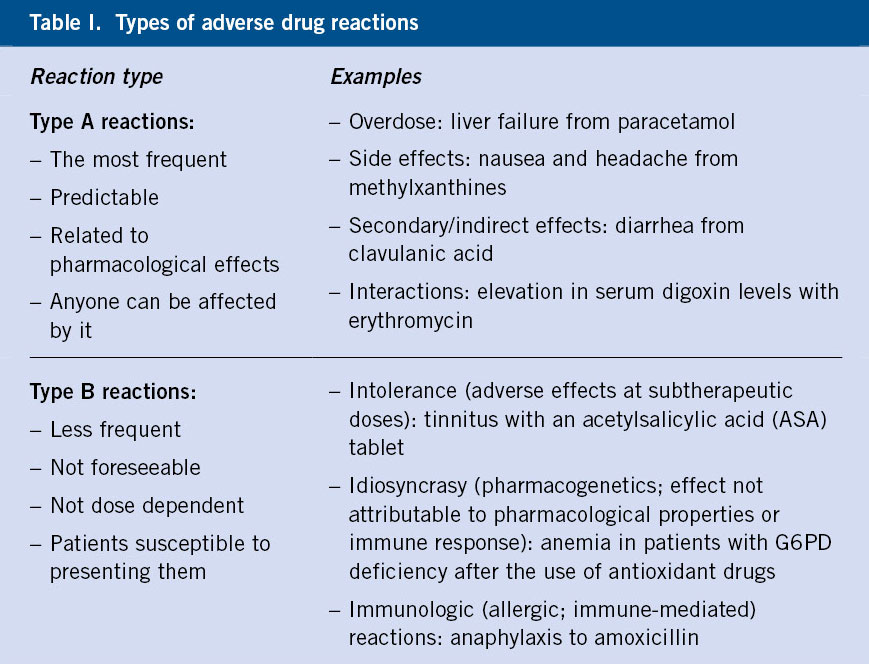
The World Health Organization (WHO) defines adverse drug reaction (ADR) as: a noxious, unwanted and unintentional response that occurs after ingesting a drug at a commonly used dose in humans to prevent, diagnose or treat a disease; as well as the responses derived from the dependence, abuse and incorrect use of medications(1). ADRs are a serious problem, with an increasing incidence as more drugs are marketed and more people are exposed to them (Table I).
Type A ADRs (more frequent) are not mediated by immunological mechanisms, but are rather predictable and related to the already known pharmacological effects of the drug itself(1,2). These reactions can occur in anyone treated and are usually mild.
Type B ADRs are less frequent, they are not predictable, and they are not related to the dose or pharmacological effect, since they are due to abnormal responses that generally occur in a small part of the population susceptible to presenting them(1,3). Within this group of reactions, there are reactions due to idiosyncrasy, intolerance and hypersensitivity to drugs. The term allergic reaction is reserved for immunologically mediated hypersensitivity reactions(4).
Classification of allergic drug reactions
ADRs can be classified according to the time of onset of symptoms, immediate or late. Depending on the pathophysiological mechanism, they are differentiated into hypersensitivity reactions: type I (immediate, mediated or not by IgE), type II (delayed, through cell lysis or cytotoxic); type III (delayed, due to immune complex deposition or complement activation); or type IV (delayed, mediated by activated T lymphocytes or cell-mediated) (Table II).
The classification of ADRs is complex, because there are multiple drugs involved and many different clinical presentations. In addition, the immunological mechanisms of some reactions are not fully defined and traditional classifications, based on the chronology between drug administration and the onset of symptom development, present difficulties.
Depending on the time of onset of symptoms, IgE-mediated early-onset reactions are considered to be those that occur between 1 and 6 hours after drug administration, and late-onset reactions are those that occur after 6 hours of taking the drug(5).
Hypersensitivity reactions are divided into four types according to the Gell and Coombs classification, which is based on the underlying pathophysiological mechanisms of each reaction. This is essential to determine the relevant diagnostic procedures, the appropriate therapeutic options, as well as to evaluate the cross-reactivity between similar drugs(2) (Table II).
• Type I: immediate reactions (mediated by IgE or non-IgE mediated). In recent years, reactions due to non-specific release or activation of mast cells have been included in this type, although these are clinically indistinguishable from an IgE-mediated reaction.
• Type II: delayed reactions, through cell lysis or cytotoxic, mediated by antibodies (generally IgG and less frequently IgM).
• Type III: delayed reactions due to deposition of immune complexes and complement activation.
• Type IV: delayed reactions, mediated by activated T lymphocytes, although they can also include other types of cells such as: macrophages, eosinophils or neutrophils. T cells are capable of generating different types of inflammatory response, depending on the cytokines produced and the other cells involved in the reaction; therefore, in turn, type IV reactions are subdivided into 4 subtypes (IVa, IVb, IVc and IVd).
Epidemiology
Drug allergy in childhood is a growing socio-sanitary problem. Although an overestimation of the epidemiology of HR to drugs is likely, the diagnosis is rarely confirmed after allergological study.
The presence of allergy to drugs in children is an important socio-sanitary problem, due to the anguish that this can cause in children, their parents and in the doctors who care for them. The absence of a correct diagnosis leads to some patients being improperly classified as allergic to drugs for an indeterminate period of time, affecting the use of second or third choice therapeutic alternatives, with higher associated healthcare costs, as well as the risk of developing antibiotic resistance(1,5). Many parents also try to avoid taking some drugs due to a family history of drug allergy(6). The epidemiology of HR to drugs is little known globally, especially in children, but it may be overestimated, since approximately 10% of parents report, at least one HR to drugs in their children(7). However, after carrying out a complete allergy study, the diagnosis of HR due to drugs is confirmed only on a few occasions(8).
The incidence of HRs varies depending on the population studied, their age, consumption patterns, diagnostic criteria, and the time elapsed until the study was carried out. Despite the fact that any drug can induce HR, in the pediatric population, antibiotics, especially those of the beta-lactam (BL) group, non-steroidal anti-inflammatory drugs (NSAIDs) and vaccines are the most frequently implicated drugs(2,8).
Fortunately, severe cutaneous adverse reactions are very rare in children, with few series published in the literature. In general, in these reactions, the most frequent drugs are BL antibiotics, NSAIDs, and antiepileptic drugs.
Risk factors
The risk of presenting HR due to drugs increases with the use of drugs and self-medication(8). In general, HR is considered to be more frequent in children diagnosed with cystic fibrosis,
which could be explained by higher levels of drug exposure, frequent use of intravenous treatments, and specific immune responses related to the disease itself(9).
Despite the fact that female gender is considered a risk factor for presenting HR, this data could not be documented in the pediatric population(8).
Infections are very frequent in children, especially viral infections, and constitute a risk factor, as well as a differential diagnosis of HR due to drugs. They may also act as cofactors in some susceptible individuals. Aminopenicillins can induce a rash, especially in children with Epstein-Barr virus (EBV) infection(8). Other viruses that are risk factors are the human immunodeficiency virus (HIV) and the cytomegalovirus (CMV).
The presence of allergic asthma and chronic urticaria are significant risk factors for NSAID reactions in children.
Clinical manifestations
The most common symptoms in adverse drug reactions in children are non-immediate maculopapular rash (MPR) and non-immediate urticaria. The differential diagnosis must mainly be done with infectious exanthema.
Immediate reactions
They can vary in severity from mild reactions to severe or very severe reactions, including anaphylaxis and anaphylactic shock.
The skin is the most frequently affected organ in the form of urticaria or MPR, with or without associated angioedema.
Respiratory involvement (essentially bronchospasm) as the only clinical manifestation is rare, being limited almost exclusively to reactions caused by NSAIDs (especially in children with a previous diagnosis of asthma) or, more frequently, as an associated symptom of an anaphylactic reaction(2,8,10).
Any IgE-mediated reaction can be life-threatening, as it can be triggered by small doses of the drug. The frequency of anaphylaxis, although considered low, seems to have increased in recent years, reaching between 5-25% according to the different series published in the literature(11).
Non-immediate reactions
They can occur even days after finalizing the treatment of the implicated drug. As in the immediate reactions, the skin is the most frequently affected organ, with MPR and non-immediate urticaria being the most frequent clinical manifestations(12,13).
Respiratory involvement, such as drug-induced pneumonitis, is exceptional in children, being observed, especially in relation to the use of chemotherapeutics.
Severe Cutaneous Adverse Reactions (SCAR) are rare in children. Among them, cases of: acute generalized exanthematous pustulosis (AGEP) have been described (acute onset appearance of sterile, non-follicular pustules <5 mm over an erythematous base); Drug Reaction with Systemic Symptoms (DRESS: rash with systemic symptoms such as: fever, adenopathies, eosinophilia and organic involvement, mainly liver); Stevens-Johnson syndrome (SJS: target lesions with vesicles and bullae associated with mucosal involvement, malaise, and fever); and toxic epidermal necrolysis (TEN: similar to SJS with greater involvement and skin detachment). The drugs most commonly implicated in these reactions are antibiotics (BL and sulfonamides, mainly), NSAIDs and anticonvulsants(2).
Serum sickness usually presents with cutaneous symptoms such as PMR or urticaria, with systemic involvement such as fever, arthritis or arthralgia, which usually appear 1-3 weeks after exposure to the drug. Its pathophysiology is unknown. It is rare, usually affects children under 5 years of age, and beta-lactams (BL), especially cefaclor, penicillin V, and amoxicillin, are the most frequently involved drugs(10).
In recent years, few cases have been described in the literature in pediatric patients presenting exclusively gastrointestinal symptoms, such as nausea, vomiting and/or diarrhea, associated with decay, approximately 2-4 hours after taking the trigger drug and that mimic the symptoms caused by food protein-induced enterocolitis (FPIES)(14). In the most severe cases there may be hypotension, lethargy or hypothermia, which can lead to hypovolemic shock. The drugs implicated in children are limited to date to amoxicillin and amoxicillin-clavulanic acid(14).
Main drugs that cause allergic reactions in children: beta-lactam antibiotics and NSAIDs
Non-steroidal anti-inflammatory drugs (NSAIDs), together with beta-lactam antibiotics, are the two leading causes of drug hypersensitivity reactions in children.
Beta-lactam antibiotics
A suspected reaction to beta-lactam antibiotics constitutes approximately 80% of consultations for drug allergy and, along with NSAIDs, they are the most common cause of allergic drug reactions in children.
Of these, aminopenicillins and, specifically, amoxicillin, occupy the first place, favored by the wide and extensive prescription of this antibiotic today.
Beta-lactams contain a common thiazolidine ring and a side chain that is responsible for the differences between each group. IgE antibodies against penicillins can be specific against the beta-lactam ring itself (common in all BL) or specific to the side chain, producing selective reactions in this case. However, it must be taken into consideration that BL from different groups may have identical side chains (cross-reactivity). Currently, the most frequent is selective allergy to the side chain without presenting a reaction with penicillin(15). However, if the allergy is caused by the major determinant of penicillin, the diagnosis is likely to be allergy to all penicillins.
There are scarce studies that analyze the cross-reactivity between penicillins and cephalosporins in children, but it has been estimated at 0.3-23.9%, being higher for cephalosporins with the same side chain(16).
Non-steroidal anti-inflammatory drugs (NSAIDs)
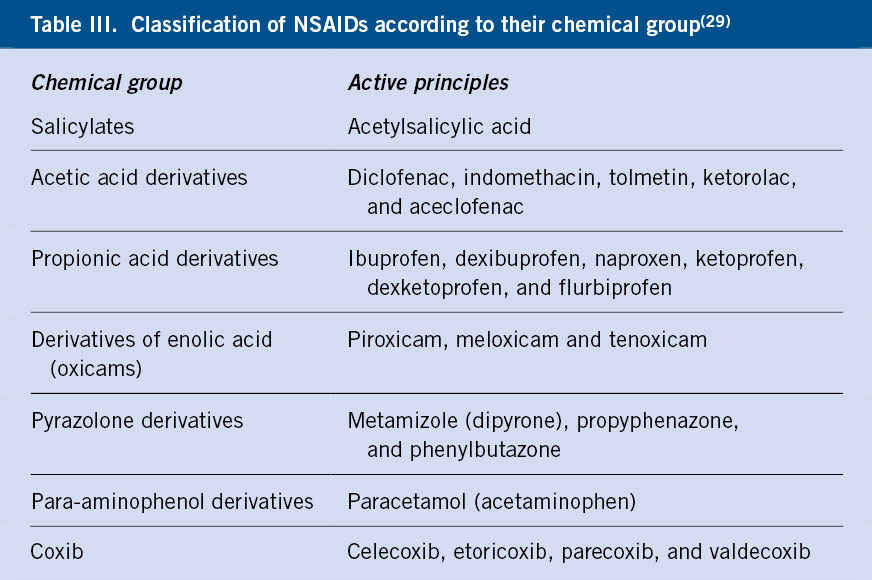
NSAIDs make up a heterogeneous group of drugs, used for their anti-inflammatory, antipyretic, analgesic and anti-aggregant action. They act by inhibiting cyclooxygenase (COX), an enzyme that catalyzes the conversion of arachidonic acid into prostaglandins. They can be classified according to their chemical composition or according to their ability to inhibit COX (Table III).
Two large groups of HR can be distinguished: selective (immunologically mediated, which usually involve a single group of NSAIDs) and non-selective or cross-intolerance (more frequent and which usually involve several groups of NSAIDs due to the relationship with the inhibition of COX-1).
Children under 10 years of age mostly present reactions due to cross-reactivity, with the presence of cofactors, such as viral infections or exercise(17).
Diagnosis
The diagnosis of allergy to drugs is based on a detailed clinical history and the allergy study using: skin tests, in vitro (specific IgE, basophil activation test and lymphocyte transformation test) and controlled exposure testing (CET) which constitutes the reference test. Allergological study is not indicated as screening without a previous reaction. Ideally, the allergy study will be performed 1-6 months after full recovery from the initial reaction, since the study loses sensitivity later on (Algorithm 1).
Clinical history
The clinical history, referred by the parents in the cases of younger children and by the patients themselves, is the first and most important step when trying to differentiate an HR from an ADR or an infection.
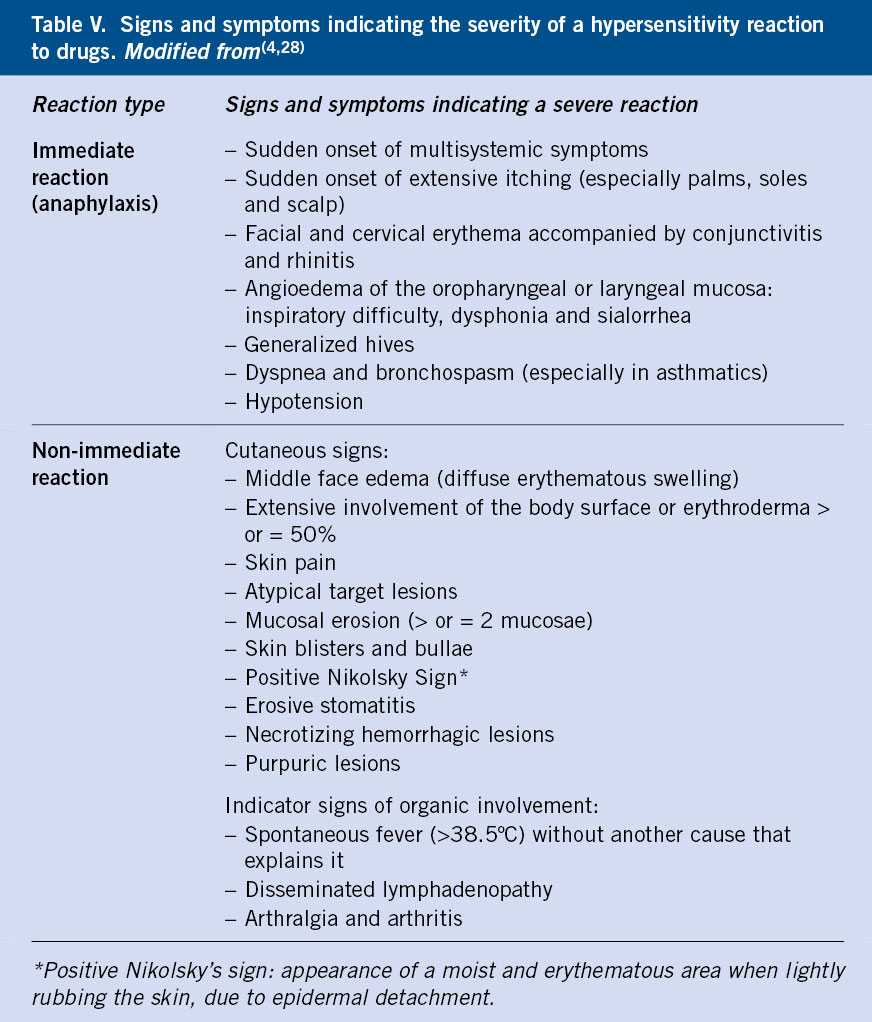
The suspicion of HR to drugs is confirmed in a few cases after performing an allergy study, and when it is, it is common that the reaction presented during the controlled exposure test does not correspond to that initially reported in the clinical history(8). This may be due to the imprecision of the data collected, the difficulty of communicating in affected children at an early age who cannot express themselves, and the time until the evaluation in consultation, which sometimes occurs years later. Tables IV and V show, respectively, the most important data in the clinical history, as well as the severity criteria that should be known, since the correct diagnostic approach will depend on it.
Skin tests
Skin tests include: intraepidermal or prick tests, intradermal (ID) tests, and epicutaneous (patch) tests. These tests are useful to identify the drugs responsible for HR and to determine possible therapeutic alternatives that are safe. However, the cost-effectiveness of skin testing varies depending on the type of reaction, drug, and time to study. On the other hand, on many occasions, its negativity does not rule out the possibility of a reaction and, in most cases, it is necessary to perform a CET to achieve a definitive diagnosis.
Prick tests are used when there is a suspicion of an immediate reaction, and they have the advantage of being safe and can be performed with drugs that are not available in sterile injectable form.
The ID tests can be used, both in immediate reactions (reading at 15 minutes) and in non-immediate reactions (reading at 24 and/or 48 hours). They are usually performed if the prick tests have been negative. However, they are invasive tests that carry a greater risk of systemic reaction, so their use in children is more limited than in adults. They are considered contraindicated in SCAR, however, some authors consider their use in a controlled setting(18).
Epicutaneous or patch tests are tests that are considered safe, although their profitability is limited, so they are only used in certain non-immediate
reactions or in SCARs as the first line in the diagnostic approach(18,19).
Beta-lactam antibiotics
Skin tests with BL antibiotics are the most studied and have the most evidence in children. However, they have low sensitivity and positive predictive value, especially in non-immediate and mild skin reactions(20).
With BL, ID tests, more sensitive than prick tests, are used primarily for immediate HR. In the case of non-immediate mild reactions with BL, the sensitivity of ID tests with late reading at 48 hours is low, although a high predictive value has been shown in case they are positive(10). Given its low profitability in this type of reaction, its use is limited to certain occasions(8,21).
Anti-inflammatory drugs
In the case of NSAIDs, the use of skin tests is limited to metamizole and, in certain cases, paracetamol, in selective HR, while there is insufficient evidence to support laboratory tests in the case of HR to NSAIDs(17).
Histology
Performing skin biopsies can be useful in severe entities such as AGEP and DRESS.
In vitro testing
• Serum tryptase during the acute and basal episode: it is used for the diagnosis of anaphylaxis. It reaches its peak between 60-90 minutes after the onset of symptoms and remains elevated for up to 5 hours. Basal tryptase determination is always necessary.
• Determination of total and specific IgE; It is available for: amoxicillin, ampicillin, penicillin G and V, cefaclor, some neuromuscular blockers and chlorhexidine(8,21). Although its sensitivity is less than 50%, it is useful in more severe immediate IgE-mediated reactions(8).
• Other in vitro tests such as: the basophil activation test (BAT) in immediate reactions, the lymphocyte transformation test (LTT) in non-immediate reactions and the quantification study of cytokines or T-/B cell products (ELISPOT), require experienced and qualified personnel, hence, they are not performed in daily clinical practice, but rather exceptionally(13).
Controlled exposure test
It is the reference test to confirm or rule out the diagnosis of HR and verify tolerance to therapeutic alternatives. It consists of the gradual administration of the suspected drug until the therapeutic dose adjusted to weight is reached. The administration guidelines vary depending on the drug used, the type of reaction and the severity of the reaction that motivates the study. They are performed when previously performed tests (skin and/or in vitro tests) have been negative, as long as the benefit to the patient outweighs the risk of the procedure.
CET should only be performed in specialized centers, where patients are carefully selected and equipment, supplies, and trained personnel are available to manage the possibility of a severe reaction during its performance(19).
CET is contraindicated in severe adverse reactions such as: anaphylaxis, SCAR or severe systemic reactions, although in certain cases, when other diagnostic procedures, such as skin or in vitro tests, are inconclusive, CET can be considered with an alternative drug, when its use is indispensable for the patient(11).
In children with a mild skin reaction such as rash (especially in late reactions), performing a CET can be considered without the need for prior skin tests, provided that the presence of any severe sign or symptom has been ruled out(8,21).
In the case of NSAIDs, CET is essential to determine the type of HR, and, when necessary, offer therapeutic alternatives.
In the case of selective reactions, the use of all NSAIDs of the responsible group will be forbidden (e.g., the use of propionic acids will be exclusively prohibited, in the case of confirming HR to ibuprofen). However, for cases of HR due to crossed intolerance, each of the authorized drugs must be confirmed by CET. In general, paracetamol at therapeutic doses (15 mg/kg/dose) is usually tolerated. Although, currently, the use of selective COX-2 inhibitors in patients under 12 years of age has not been approved, several studies support the safety of the use of: etoricoxib, celecoxib and meloxicam as a weak COX-1 inhibitor, in pediatric patients as an alternative(22). Other proposed alternatives to the use of NSAIDs are corticosteroids, powerful anti-inflammatories, together with physical measures to reduce temperature, and opiates as analgesics.
The diagnostic management of HR by BL is summarized in Algorithm 2.
Differential diagnosis
In most cases, it is impossible to distinguish between a rash induced by an infectious process and one that is secondary to drugs. Most skin reactions that occur while undergoing treatment with a BL are due to the infection itself, and HR has been confirmed in less than 10% of cases(8). In fact, the incidence of rash in children is estimated at 153.8/10,000 children and it can be caused: by the infection itself, by the interaction of the drug with the infection, or because it truly is an allergic reaction to the drug(23). The infections that most frequently cause this type of affectation are: EBV, CMV, parvovirus or Mycoplasma(8,24). A detailed clinical history, together with serological tests or virus detection tests by means of PCR in the acute phase, will allow in certain cases, to differentiate an infectious exanthema from an HR. The diagnosis must be made during a minimum of 4-6 weeks after the complete resolution of all clinical symptoms, to avoid both false positives and false negatives(4).
In the differential diagnosis of DRESS, diseases that occur with: fever, rash, lymphadenopathy and involvement of other organs, such as infections by: EBV, parvovirus B19, measles, dengue or Coksackie virus must be taken into account(25).
It is important to differentiate anaphylactic reactions from other clinical manifestations that may occur during drug administration, such as vasovagal syndromes or the well-known oculorespiratory syndrome, described after administration of influenza virus and characterized by: bilateral conjunctivitis, facial edema, and upper pathway respiratory symptoms(11).
Re-evaluation
The natural history of drug hypersensitivity with most classes of drugs in children is largely unknown. There is also anecdotal information that some children with NSAID-exacerbated skin disease may resolve their HR to NSAIDs when the urticarial episodes subside. For this reason, it is advisable to periodically re-evaluate these patients and assess the possibility of performing a CET always in a hospital setting and with trained personnel(15).
Treatment
In the event of an adverse drug reaction, it is essential to withdraw the suspected drug and avoid any drug that belongs to the same group, as well as to recognize severe reactions that require urgent action (Table V).
Immediate reactions
In the case of mild immediate reactions, such as urticaria or angioedema, antihistamines, preferably second generation, will be administered as the first option. An immediate severe reaction or anaphylaxis is considered life-threatening, which requires, as the first line of treatment, the administration of intramuscular adrenaline (0.01 mg/kg up to 0.5 mg maximum dose), a dose that can be repeated after 5 -15 minutes, if the manifestations persist. In the event of cardiorespiratory arrest, resuscitation maneuvers will be started and other support measures will be added. It should be noted that the administration of systemic antihistamines and corticosteroids should not be used as first-line treatment in these cases or in monotherapy, since they do not reverse laryngeal edema, bronchospasm, or hypotension(26).
Non-immediate reactions
In mild non-immediate reactions, antihistamines may be used, however, in uncomplicated MPR the use of corticosteroids is not recommended.
SCARs require multidisciplinary treatment and, sometimes, admission to intensive care units. Although there is no clear consensus, in DRESS cases, systemic corticosteroids (prednisolone 1 mg/kg/day) are the mainstay treatment in the acute phase in patients with severe organic involvement. Other drugs used are cyclosporine or anti-TNF(19).
Desensitization
In confirmed cases of allergy to a drug in which its administration is essential for the patient, desensitization can be considered. However, the experience in children is scarce and protocols adapted for adults are often used(27).
For children with positive allergy tests after suspected anaphylaxis to anti-infective vaccines, this can be administered in graduated doses according to the protocol proposed by the American Academy of Pediatrics(11).
Role of the Primary Care pediatrician
The Primary Care physician plays a determining role when faced with a patient with suspected HR to a drug (Table VI), both for the correct diagnosis of HR and to avoid overdiagnosis that is harmful to the patient. The first step will be to rule out that it is an ADR and, in case of suspicion of HR, refer the patient to the Allergology Service with a detailed clinical history.
His role entails:
• To identify a possible hypersensitivity reaction.
• To differentiate it from a type A reaction.
• To thoroughly collect all the necessary elements for an adequate filiation of the reaction (Table IV).
• To know the severity criteria (Table V).
• To manage a reaction in the acute phase, either applying the appropriate pharmacological treatment or referring to the Emergency Department (treatment of anaphylaxis).
• To discontinue the suspect drug and maintain the prohibitions regarding the use of drugs from the same family or group, until its assessment in the Allergology Service.
• To adequately inform the patient about the drugs to avoid and the alternatives that can be used until the completion of the allergy study.
• To inform patients that an allergy study is not indicated for screening(28).
Conflict of interests
There is no conflict of interest in the preparation of this manuscript. Declaration of interests: none.
Bibliography
The asterisks reflect the interest of the article in the opinion of the authors.
1. Rukasin CRF, Norton AE, Broyles AD. Pediatric Drug Hypersensitivity. Curr Allergy Asthma Rep. 2019; 19: 11. Available at: https://doi.org/10.1007/s11882-019-0841-y.
2. Park JS, Suh DI. Drug Allergy in Children: What Should We Know? Clin Exp Pediatr. 2020; 63: 203-10. Available at: https://doi.org/10.3345/kjp.2019.00675.
3. Trubiano JA, Stone CA, Grayson ML, Urbancic K, Slavin MA, Thursky KA, et al. The 3 Cs of Antibiotic Allergy-Classification, Cross-Reactivity, and Collaboration. J Allergy Clin Immunol Pract. 2017; 5: 1532-42. Available at: https://doi.org/10.1016/j.jaip.2017.06.017.
4. Demoly P, Adkinson NF, Brockow K, Castells M, Chiriac AM, Greenberger PA, et al. International Consensus on drug allergy. Allergy. 2014; 69: 420-37. Available at: https://doi.org/10.1111/all.12350.
5. Castells M. Drug allergy: Come, vidi, vici-come, understand, and delabel, avoid, or desensitize. Ann Allergy Asthma Immunol. 2019; 123: 1-2. Available at: https://doi.org/10.1016/j.anai.2019.05.010.
6. Stone CA, Trubiano J, Coleman DT, Rukasin CRF, Phillips EJ. The challenge of de‐labeling penicillin allergy. Allergy. 2020; 75: 273-88. Available at: https://doi.org/10.1111/all.13848.
7. Erkoçoğlu M, Kaya A, Civelek E, Özcan C, Çakır B, Akan A, et al. Prevalence of confirmed immediate type drug hypersensitivity reactions among school children. Pediatric Allergy Immunol. 2013; 24: 160-7. Available at: https://doi.org/10.1111/pai.12047.
8.*** Gomes ER, Brockow K, Kuyucu S, Saretta F, Mori F, Blanca-López N, et al. Drug hypersensitivity in children: Report from the pediatric task force of the EAACI Drug Allergy Interest Group. Allergy. 2016; 71: 149-61. Available at: https://doi.org/10.1111/all.12774.
9. Whitaker P. Naisbitt D, Peckham D. Nonimmediate β-lactam reactions in patients with cystic fibrosis. Curr Opin Allergy Clin Immunol. 2012; 12: 36975. Available at: https://doi.org/10.1097/ACI.0b013e328355b849.
10. Blanca‐López N, Atanaskovic‐Markovic M, Gomes ER, Kidon M, Kuyucu S, Mori F, et al. An EAACI Task Force report on allergy to beta‐lactams in children: Clinical entities and diagnostic procedures. Pediatric Allergy and Immunology. 2021; 32: 1426-36. Available at: https://doi.org/10.1111/pai.13529.
11. Atanaskovic-Markovic M, Gomes E, Cernadas JR, du Toit G, Kidon M, Kuyucu S, et al. Diagnosis and management of drug-induced anaphylaxis in children: An EAACI position paper. Pediatric Allergy Immunol. 2019; 30: 269-76. Available at: https://doi.org/10.1111/pai.13034.
12. Brockow K, Ardern-Jones MR, Mockenhaupt M, Aberer W, Barbaud A, Caubet JC, et al. EAACI position paper on how to classify cutaneous manifestations of drug hypersensitivity. Allergy. 2019; 74: 14-27. Available at: https://doi.org/10.1111/all.13562.
13. Romano A. Atanaskovic‐Markovic M, Barbaud A, Bircher AJ, Brockow K, Caubet J, et al. Towards a more precise diagnosis of hypersensitivity to beta‐lactams – an EAACI position paper. Allergy. 2020; 75: 130015. Available at: https://doi.org/10.1111/all.14122.
14. Mori F.Liccioli G, Fuchs O, Barni S, Giovannini M, Sarti L, et al. Drug‐induced enterocolitis syndrome: Similarities and differences compared with food protein‐induced enterocolitis syndrome. Pediatric Allergy Immunol. 2021; 32: 1165-72. Available at: https://doi.org/10.1111/pai.13491.
15. López NB, Cornejo-García, JA, Pérez-Alzate D, Pérez-Sánchez N, Plaza-Serón MC, Doña I, et al. Hypersensitivity Reactions to Nonsteroidal Anti-inflammatory Drugs in Children and Adolescents: Selective Reactions. J Investig Allergol Clin Immunol. 2015; 25: 385-95.
16. Pfaar O, Bastl K, Berger U, Buters J, Calderon MA, Clot B, et al. Defining pollen exposure times for clinical trials of allergen immunotherapy for pollen-induced rhinoconjunctivitis-an EAACI position paper. Allergy. 2017; 72: 713-22. Available at: https://doi.org/10.1111/all.13092.
17. Kidon M, Blanca-López N, Gomes E, Terreehorst I, Tanno L, Ponvert C, et al. EAACI/ENDA Position Paper: Diagnosis and management of hypersensitivity reactions to non-steroidal anti-inflammatory drugs (NSAIDs) in children and adolescents. Pediatric Allergy Immunol. 2018; 29: 469-80. Available at: https://doi.org/10.1111/pai.12915.
18. Barbaud A, Castagna J, Soria A. Skin tests in the work-up of cutaneous adverse drug reactions: A review and update. Contact Dermatitis. 2022; 86: 344-56. Available at: https://doi.org/10.1111/cod.14063.
19. De Groot AC. Patch testing in drug reaction with eosinophilia and systemic symptoms (DRESS): A literature review. Contact Dermatitis. 2022; 86: 443-79. Available at: https://doi.org/10.1111/cod.14090.
20. Ponvert C, Perrin Y, Bados-Albiero A, le Bourgeois M, Karila C, Delacourt C, et al. Allergy to beta-lactam antibiotics in children: results of a 20-year study based on clinical history, skin and challenge tests. Pediatric Allergy Immunol. 2011; 22: 4118. Available at: https://doi.org/10.1111/j.1399-3038.2011.01169.x.
21. Ibáñez MD, Rodríguez del Río P, Lasa EM, Joral A, Ruiz-Hornillos J, Muñoz C, et al. Prospective assessment of diagnostic tests for pediatric penicillin allergy. Ann Allergy Asthma Immunol. 2018; 121: 235-44.e3. Available at: https://doi.org/10.1016/j.anai.2018.05.013.
22. Corzo JL, Zambonino MA, Muñoz C, Mayorga C, Requena G, Urda A, et al. Tolerance to COX-2 inhibitors in children with hypersensitivity to nonsteroidal anti-inflammatory drugs. Br J Dermatol. 2014; 170: 725-9. Available at: https://doi.org/10.1111/bjd.12674.
23.*** Tsabouri S, Atanaskovic-Markovic M. Skin eruptions in children: Drug hypersensitivity vs viral exanthema. Pediatric Allergy Immunol. 2021; 32: 824-34. Available at: https://doi.org/10.1111/pai.13485.
24. Bernaola M, Morales-Cabeza C, Ibáñez-Sandin MD. De-labeling Penicillin Allergy in Pediatric Population. Curr Treat Options Allergy. 2022; 9: 234-49. Available at: https://doi.org/10.1007/s40521-022-00315-4.
25. Cabañas R, Ramirez E, Sendagorta E, Alamar R, Barranco R, Blanca-López N, et al. Spanish guidelines for diagnosis, management, treatment, and prevention of dress syndrome. J Investig Allergol Clin Immunol. 2020; 30: 229-53. Available at: https://doi.org/10.18176/jiaci.0480.
26. Nurmatov UB, Rhatigan E, Simons FER, Sheikh A. H2-antihistamines for the treatment of anaphylaxis with and without shock: a systematic review. Ann Allergy Asthma Immunol. 2014; 112: 12631. Available at: https://doi.org/10.1016/j.anai.2013.11.010.
27. Rodríguez del Río P, Andión M, Ruano D, Escudero C, Méndez Brea P, Sánchez-García S, et al. Initial experience with carboplatin desensitization: A case series in a pediatric hospital. Pediatric Allergy and Immunology. 2018; 29: 111-5.
28.*** Doña I, Caubet JC, Brockow K, Doyle M, Moreno E, Terreehorst I, et al. An EAACI task force report: Recognizing the potential of the primary care physician in the diagnosis and management of drug hypersensitivity. Clin Transl Allergy. 2018; 8: 16. Available at: https://doi.org/10.1186/s13601-018-0202-2.
29. Ortega Rodríguez NR, Doña Díaz I, Blanca López N, López San Martín M, Muñoz Román C. Hipersensibilidad a los antiinflamatorios no esteroideos. Hypersensitivity to non-steroidal anti-inflammatory drugs. In: Dávila González IJ, Jáuregui Presa I, Olaguibel Rivera JM, Zubeldia Ortuño JM, eds. Tratado de Alergología. Ergon. 2016. p. 1551-67.
30. Armentia Medina A, Martín Armentia S. Alergia a medicamentos y drogas. Allergy to medications and drugs. Pediatr Integral. 2018; XXII: 147-54.
Recommended bibliography
– Gomes ER, Brockow K, Kuyucu S, Saretta F, Mori F, Blanca-López N, et al. Drug hypersensitivity in children: Report from the pediatric task force of the EAACI Drug Allergy Interest Group. Allergy. 2016; 71: 149-61. Available at: https://doi.org/10.1111/all.12774.
Extensive review on the diagnosis and management of HR, specifically in the pediatric population.
– Tsabouri S, Atanaskovic-Markovic M. Skin eruptions in children: Drug hypersensitivity vs viral exanthema. Pediatric Allergy Immunol. 2021; 32: 824-34. Available in: https://doi.org/10.1111/pai.13485.
Recommended to deepen knowledge about the differential diagnosis of maculopapular rash.
– Doña I, Caubet JC, Brockow K, Doyle M, Moreno E, Terreehorst I, et al. An EAACI task force report: Recognising the potential of the primary care physician in the diagnosis and management of drug hypersensitivity. Clin Transl Allergy. 2018; 8: 16. Available at: https://doi.org/10.1186/s13601-018-0202-2.
It presents in a practical way the management of HR from Primary Care perspective. This is especially recommended.
| Clinical case |
|
A 3-year-old girl had a history of hospital admission for 5 days due to bronchiolitis. No family history of interest. She was referred to the Allergology Department due to suspected allergy to amoxicillin. In February 2022, after the diagnosis of acute otitis media, treatment was prescribed with amoxicillin 50 mg/kg/day (250 mg every 8 hours) for 7 days, and ibuprofen 5 mg/kg/dose (75 mg every 8 hours) in case of fever or pain. Twenty-four hours after finishing the fifth day of antibiotic treatment, she required assistance in the Emergency Department due to presentation of skin lesions when she got up in the morning that were described as: a non-pruritic maculopapular rash affecting the trunk, back and lower limbs, and not affecting soles of the feet nor palms of the hands. The last dose of amoxicillin before the onset of symptoms had been at least 8 hours before (the girl went to bed without skin lesions) and the last dose of ibuprofen had been 48 hours before the onset of symptoms. The rest of the physical examination showed no abnormalities. There were no mucosal lesions and the patient remained afebrile and in good general condition. In the Emergency Department, she was administered a dose of dexchlorpheniramine 0.2 mg/kg/day and home treatment was prescribed with the same antihistamine syrup for 4 days, and treatment with amoxicillin was withdrawn. She was diagnosed with a rash probably secondary to amoxicillin vs infectious, she was told to avoid beta-lactam antibiotics and she was referred to Allergology. Symptoms resolved in approximately 3 days with no desquamation or residual lesions. Previously, the patient had tolerated amoxicillin and cefuroxime without incidents and, after the episode described, she had avoided taking amoxicillin and other beta-lactam antibiotics, but she had resumed tolerating ibuprofen without issues. Given the characteristics, mild and, a priori, a late reaction (onset of symptoms hours after taking the most suspicious drug, amoxicillin), the drug allergy study was planned and the corresponding informed consent was signed. Prick or intradermal skin tests were not performed, nor was total and specific IgE determined, considering the profitability of these complementary tests in this type of reaction. In the absence of alarm signs (mucosal lesions, absence of fever, no residual lesions or desquamation and resolution of symptoms in three days), a controlled exposure test with amoxicillin was performed directly, with a single dose of 250 mg, being negative. Home treatment with amoxicillin 250 mg every 12 hours for 5 days was also prescribed and, subsequently, tolerance to said drug was confirmed and, therefore, the diagnosis of allergy to amoxicillin was ruled out, confirming that the rash previously presented was of infectious origin. No controlled exposure test was performed with ibuprofen, since the patient had tolerated it after the episode that prompted the allergological study.
|
 Allergy to medicines and drugs
Allergy to medicines and drugs