|
| Topics on Continuous Training |
V.M. García Nieto*, T. Moraleda Mesa**, P. Tejera Carreño***
*Section of Pediatric Nephrology, Nuestra Señora de Candelaria University Hospital, Santa Cruz de Tenerife. **Pediatrics Department, Poniente University Hospital, El Ejido. Almeria. ***Section of Pediatric Nephrology, Maternal and Child University Hospital, Las Palmas de Gran Canaria
| Abstract
Renal lithiasis is a multifactorial pathological situation where alteration of the natural conditions of urine crystallization occurs. There are substances in the urine that, when elevated, favor the formation of stones (promoters: calcium, oxalate, uric acid, cystine) and others that will do so if they are reduced (inhibitors: citrate, magnesium). The metabolic diagnosis can be made in 24-hour urine or by determining urinary ratios in the first urine of the day. Prelithiasis is a situation where stones have not yet formed, but where a metabolic abnormality potentially causing stone formation is detected. Prelithiasis may present as macro- or microscopic hematuria, sterile leukocyturia, dysuria, pollakiuria, urinary urgency, nocturnal enuresis, recurrent abdominal pain, mild proteinuria and urinary tract infections. Morphologically, crystalluria may be seen in the urinary sediment or mobile hyperechogenic particles on bladder ultrasound. The calcium/citrate ratio is a good argument for determining whether there is an elevated lithogenic risk. Treatment will be based primarily on a series of protective dietary guidelines and, if clinical symptoms do not improve or if stones are present, pharmacological measures (thiazides, citrate salts, bisphosphonates) will be chosen. |
| Resumen
La litiasis renal es una situación patológica multifactorial donde se produce una alteración de las condiciones naturales de cristalización de la orina. Hay sustancias en la orina que, al estar elevadas, favorecen la formación de cálculos (favorecedores: calcio, oxalato, ácido úrico, cistina) y otras que lo harán en el caso de encontrarse reducidas (inhibidores: citrato, magnesio). El diagnóstico metabólico se puede realizar en orina de 24 horas o determinando los cocientes urinarios en la primera orina del día. La prelitiasis es aquella situación en la que aún no se han formado los cálculos, pero en la que se detecta una anomalía metabólica potencialmente causante de su formación. La prelitiasis puede presentarse en forma de hematuria macro o microscópica, leucocituria estéril, disuria, polaquiuria, urgencia miccional, enuresis nocturna, dolor abdominal recurrente, proteinuria discreta e infecciones urinarias. Morfológicamente, puede observarse cristaluria en el sedimento urinario o partículas hiperecogénicas móviles en la ecografía vesical. El cociente calcio/citrato es un buen argumento para determinar si existe un riesgo litogénico elevado. El tratamiento se basará fundamentalmente en una serie de normas dietéticas protectoras y, en el caso de no mejorar la sintomatología clínica o de presentar cálculos, se optará por medidas farmacológicas (tiazidas, sales de citrato, bifosfonatos). |
Key words: Idiopathic hypercalciuria; Hypocitraturia; Prelithiasis; Lithogenic risk.
Palabras clave: Hipercalciuria idiopática; Hipocitraturia; Prelitiasis; Riesgo litiásico.
Pediatr Integral 2022; XXVI (8): 492 – 500
OBJECTIVES
• To comprehend the morphological and metabolic bases of renal lithiasis in childhood.
• To understand which factors favor stone formation and which ones are protective.
• To learn the renal functional methods necessary to identify if the concentration of a substance involved in urolithiasis is altered.
• To recognize the symptoms and signs associated with prelithiasis in children and the best way to make its diagnosis.
• To make a proper therapeutic approach from the beginning of the follow-up of patients with lithiasis or prelithiasis.
• To know the meaning and importance of calciuria and citraturia determination.
Hypercalciuria and hypocitraturia. The concept of prelithiasis in Pediatrics
Nephrolithiasis
Renal lithiasis is a multifactorial pathological situation. Stones can be produced by: an excessive concentration of promoting substances, by a reduction in the concentration of crystallization inhibitors, by the existence of heterogeneous nucleation and by the existence of renal cavities with low urodynamic efficiency. If the calculus is available, its chemical composition should be studied. In addition, the urinary concentration of calcium, oxalate, uric acid, cystine, magnesium, and citrate should be determined.
General information
Renal lithiasis can be defined as an alteration of the natural conditions of urine crystallization. The factors involved in the formation of crystals can be very diverse and, for this reason, renal lithiasis is a multifactorial pathological situation. The time required to generate a crystal depends fundamentally: on its own nature, on the supersaturation of the solution (excess solute in the solution: driving force for crystallization), on the presence of pre-existing solid particles (so-called heterogeneous nucleation) and the presence of crystallization inhibitors. The latter are substances that, due to their chemical structure, interact with the nucleus or the faces of the crystal, significantly interfering in its formation or/and development, reducing or preventing crystallization processes. All human urines are supersaturated with respect to calcium oxalate(1), in such a way that the degree of supersaturation is higher in individuals with hypercalciuria and/or hyperoxaluria. Human urine can also contain a wide variety of heterogeneous nucleation agents, such as: protein aggregates, altered renal epithelia, or bacteria. It is obvious that time is a very important variable in these crystal formation processes. Thus, it is evident that by increasing the length of time spent by the urine in the urinary tract (mainly in the upper tract), the possibility of crystallization processes leading to the formation of kidney stones increases. The existence of renal cavities with low urodynamic efficiency is an important risk factor for the development of stones. In fact, it has been shown that morphoanatomical factors can play an important role in calculogenesis. Hence, it would explain why a relapsed patient, in whom it is assumed that the urine will have the same composition in both kidneys, only forms stones in one of them(1). When crystal development occurs in the urinary bladder, these are usually shed without difficulty in the form of asymptomatic crystalluria.
Classification of kidney stones
Each type of stone is formed due to specific etiological factors that can be deduced from its composition, as well as its macro and microstructure(1).
Regardless of their chemical composition, kidney stones can be broadly classified into two broad categories: stones formed on the renal walls (attached to the papillae), in which the zone of attachment to the epithelium is clearly distinguished, and stones developed in the renal cavities (without attachment zone to the epithelium).
Briefly, the usual composition and frequency of calculi is as follows(1):
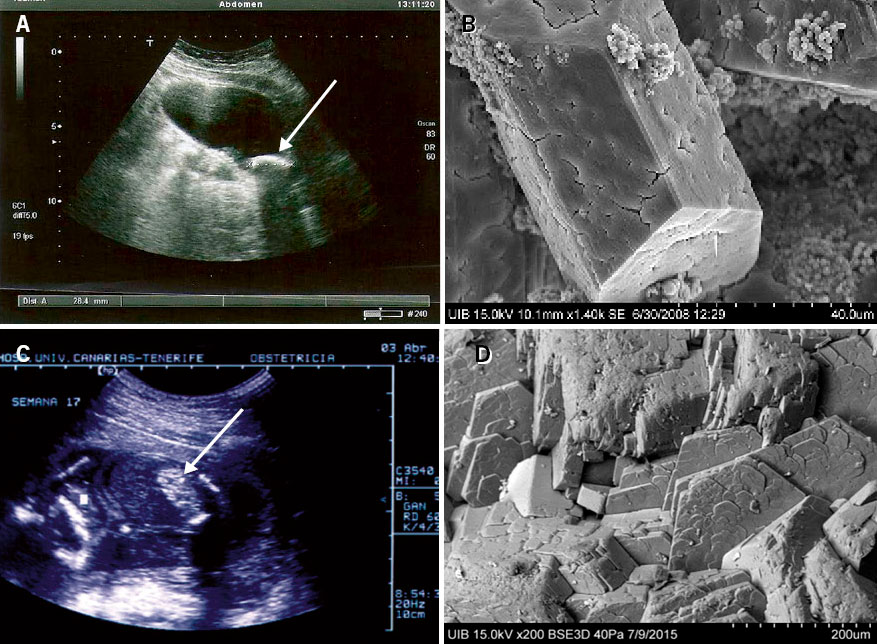
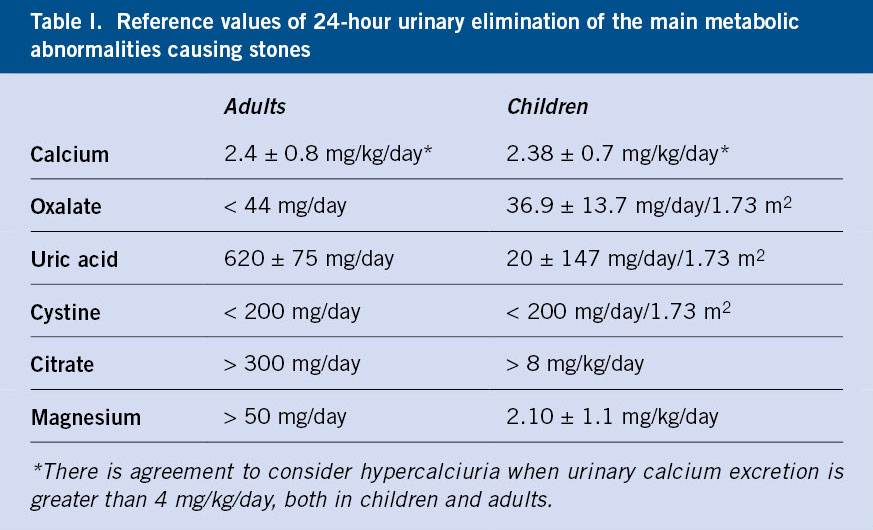
• Calcium oxalate dihydrate stones (33.8 % of lithiasis). They are especially observed in patients with hypercalciuria and/or with a high calcium/citrate ratio(2) (Figs. 1A and 1B).
Figure 1. Patient with idiopathic hypercalciuria: A. Ultrasound calculus with typical sonic shadow (white arrow). B. Calculus of calcium oxalate dihydrate. Scanning electron microscope view with the typical spearhead morphology. Its surface shows signs of the start of dissolution in a process of transformation into calcium oxalate monohydrate. Patient with type 1 oxalosis: at 23 months of age, she had acute obstructive renal failure due to bilateral stones that required bilateral double J stent placement. C. Stone embedded in the vesicoureteral junction (black arrow). D. Calculus of calcium oxalate monohydrate. The typical compact columnar structure characteristic of these cases is observed. Electron microscopy images from the Laboratori d’Investigació en Litiasi Renal, Universitat de les Illes Balears, Palma de Mallorca (Dr. Félix Grases).
• Calcium oxalate monohydrate stones of cavities (16.4 % of lithiasis). They form in areas of the urinary tract where urine remains in a state of stasis (cavities with low urodynamic efficiency) together with hyperoxaluria (Figs. 1C and 1D) or a deficiency of crystallization inhibitors.
• Calcium oxalate monohydrate stones of papillae (12.9 % of lithiasis). They are associated with hyperoxaluria or a deficiency of crystallization inhibitors.
• Uric acid stones (8.2 % of lithiasis) or mixed calcium oxalate monohydrate and uric acid stones (2.6 % of lithiasis). They are seen in patients with acid urine and hyperuricosuria.
• Calculi of hydroxyapatite (calcium phosphate) (7.1 % of lithiasis) or calcium oxalate dihydrate and hydroxyapatite (11.2 % of stones). These are generated in patients with alkaline urine, hypercalciuria and/or deficiency of crystallization inhibitors, such as hypocitraturia (elevated calcium/citrate ratio)(2), particularly in renal tubular acidosis.
• Calculi of magnesium ammonium phosphate or struvite (4.1 % of lithiasis). These are produced in urinary infections caused by ureolytic germs, such as Proteus mirabilis (Figs. 2A and 2B).
Figure 2. Patient diagnosed at birth with myelomeningocele in whose follow-up presented several positive urine cultures for Proteus mirabilis and Citrobacter: A. Bladder stone (white arrow) that required surgical removal. B. Large crystals of magnesium ammonium phosphate (struvite) and some areas of hydroxyapatite spherulites were observed. On the faces of the struvite crystal the “Y” marks are observed, allowing its rapid identification. Child with cystinuria: C. During pregnancy, fetal ultrasound check-ups detected hyperechogenicity of the colon (white arrow) in the absence of other intestinal anomalies, compatible with cystine crystals. D. Cystine crystals with the typical hexagonal morphology. Electron microscopy images from the Laboratori d’Investigació en Litiasi Renal, Universitat de les Illes Balears, Palma de Mallorca (Dr. Félix Grases).
• Cystine stones (1.1 % of lithiasis) typical of simple proximal tubulopathy, cystinuria (Figs. 2C and 2D).
Stone-causing metabolic abnormalities
In clinical practice, the concentrations of: calcium, uric acid, oxalate, cystine, citrate and magnesium are usually determined. The first four favor the formation of stones when their concentration in the urine is high (promoters). On the other hand, citrate and magnesium favor their formation when their urinary amounts are reduced (inhibitors). Although they are not determined in daily practice, in addition to citrate and magnesium, other substances such as pyrophosphate, certain glycosaminoglycans, nephrocalcin and phytate, act by inhibiting the formation of calcium oxalate and calcium phosphate crystals. Phytate, present in the husk of cereals and legumes, is a powerful inhibitor of crystallization.
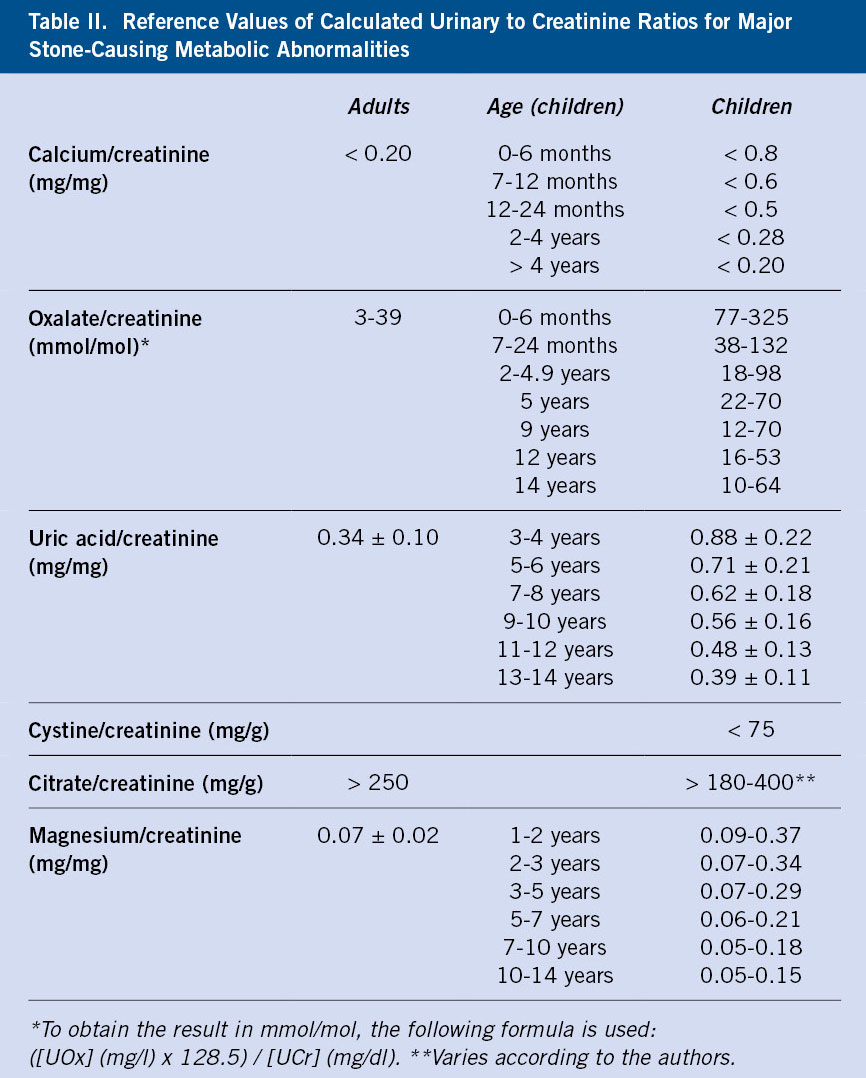
The solutes mentioned above can be measured in 24-hour urine (Table I) or in isolated urine samples.
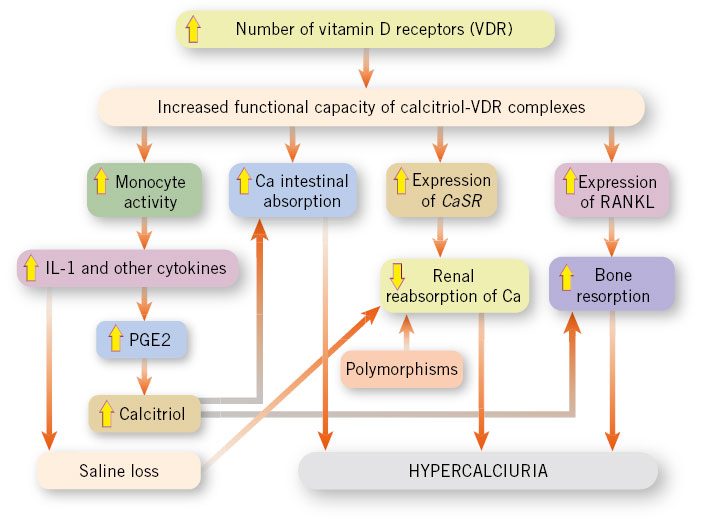
It should be confirmed that the hourly urinary collection is correct by calculating the urinary creatinine clearance (normal: 14-26 mg/kg/day). Currently, urinary quotients are increasingly used due to their ease in collecting samples, especially in childhood, and because the concentration, especially of calcium and citrate, can vary at different times of the day(3). For this reason, some of us prefer to determine in children, urinary quotients in two urine samples collected at two periods, namely, at night (before dinner) and in the first urine of the morning. In any case, urinary quotients are very useful, especially in the follow-up of patients (Table II). In the particular case of uric acid, the calculation of the excretion rate has been proposed, so it must be determined both in blood and urine. The formula is: ([uric acid]u × [creatinine]p)/[creatinine]u. Values higher than 0.53 mg/100 ml RGF (renal glomerular filtration rate) are considered high.
Idiopathic hypercalciuria
Idiopathic hypercalciuria is the most common cause of stones, both in children and in adults. Urinary calcium concentration should be elevated in the absence of hypercalcemia. It can manifest in the form of lithiasis, but it is frequently associated with various symptoms (prelithiasis) such as: hematuria, micturition symptoms, urinary infection and abdominal pain. The cause has been much debated for decades, but it is probably due to an increase in the number of receptors for vitamin D. Renal ultrasound is mandatory, but bone densitometry is not. It is important to know that it is not a disease, but rather a metabolic abnormality. For this reason, treatment must be dietary, trying to increase the urinary concentration of protective factors and reduce that of calcium.
Definition
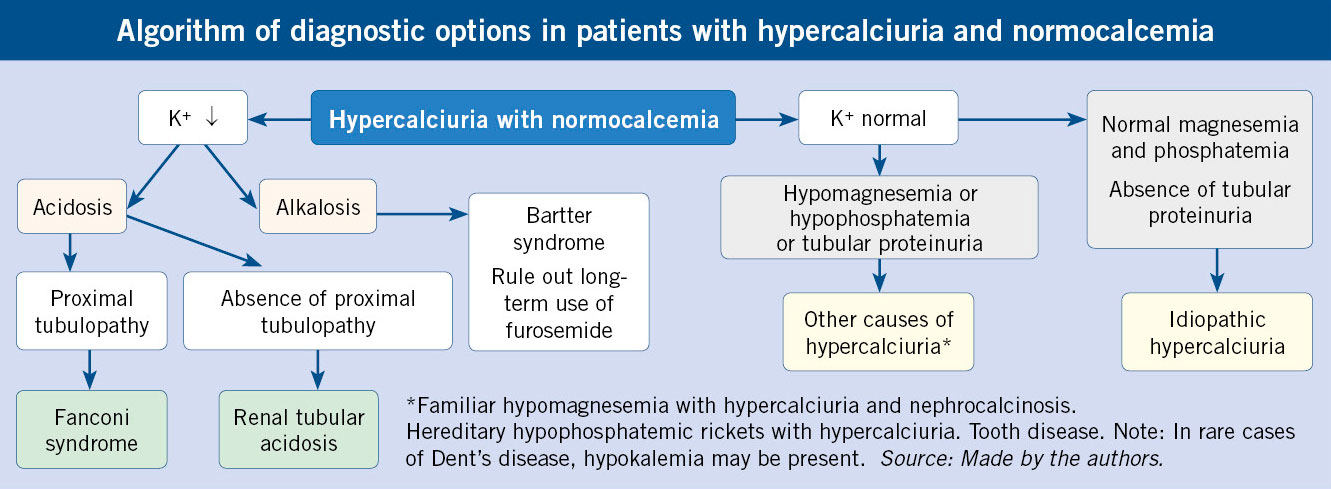
Idiopathic hypercalciuria (IH) is defined as that clinical situation in which an increase in urinary calcium excretion is verified, in the absence of hypercalcemia and other known causes of hypercalciuria (Algorithm). It is the most common cause of renal lithiasis, both in children and adults (around 40 % in series of children and 60 % in adult series). IH is one of the most frequent metabolic abnormalities in humans, and its prevalence rates have been reported in the healthy population, depending on the country, between 0.6 and 12.5 %. In Spain, the prevalence rates described range from 3.8 to 7.8 %(4).
Clinical presentation
Initially, IH was only associated with renal colic and stone expulsion. In recent decades, its diagnosis has been much more frequent than before, because it is now known that it can present in the pediatric age with very diverse symptoms in the absence of kidney stone formation. In this sense, in 1981, three different pediatric groups published that gross or microscopic hematuria could be a manifestation of IH and, therefore, be a precursor of the potential capacity to suffer renal colic years later. The author of the third of these studies, Moore, recorded that children affected by IH could present, apart from lithiasis, with other symptoms or signs such as: dysuria, sterile leukocyturia, nocturnal enuresis, frequency, urinary urgency, and even slight proteinuria(5). Shortly after, the association between urinary tract infection (UTI) and IH was published(6).
Subsequently, the relationship between microhematuria or macroscopic hematuria (blood color and, on many occasions, at the end of urination) and the presence of IH was confirmed in various studies, so much so that, 25 to 42 % of the children sent to referral centers for hematuria have IH. However, hematuria is not specific to IH, since other metabolic abnormalities causing stone disease, such as hyperuricosuria or hyperoxaluria, may be associated with hematuria. The production of experimental hypercalciuria for a short time is not accompanied by hematuria, so it is assumed that any metabolic abnormality causing lithiasis can produce microcrystals, a lesion of the renal tubular epithelium, and secondary hematuria. In addition, a group from Hospital 12 de Octubre, in Madrid, has described the association of hypercalciuria, hyperuricosuria and nephrolithiasis with nephropathy of thin basement membranes(7). The presence of recurrent abdominal pain “not typical of renal colic” has also been associated with IH.
The association between IH and UTI deserves a special mention. In 1987, Cervera et al., members of the Gregorio Marañón Hospital, in Madrid, published that the frequency of UTI in their patients with IH was 48.9 %(6), which contrasts with the estimated prevalence of UTI in the general population, in order of 1-2 % in boys and 3-5 % in girls. These initial data were subsequently confirmed in various studies. In the same way and conversely, it has been described that the frequency of hypercalciuria in series of children with UTI oscillates between 20 and 44 %. In a recent work published by our Group, we have observed that the frequency of prelithiasis (including the calculation of the calcium/citrate ratio) in children with UTI due to Escherichia coli was 47.5 % and there was a positive family history for urolithiasis in 68.3 % of cases(8). In this article we postulate that children with prelithiasis must somehow have a reduced constitutive defensive capacity against these bacteria. Subsequently, our observation of a higher frequency of renal scars in children with prelithiasis is very significant, in relation to those with a normal urinary metabolic study(8).
Pathogenic theories. Hypercalciuric rats
During the last third of the former century, different pathogenic theories of IH were enunciated trying to explain it based on the existence of a renal tubular urinary loss of calcium, an increase in its intestinal absorption, or an increase in its bone reabsorption, in addition to a dietary origin linked to an excess in the intake of sodium and/or protein. All these theories have been reviewed in a recent publication signed by our Group(9). In 1990, Pacifici et al. demonstrated that blood monocytes isolated from patients with IH produced an increased amount of cytokines, such as: interleukin-1α (IL-1), granulocyte-macrophage colony-stimulating factor, and tumor necrosis factor-α (TNF-α). An increase in the activity of these cytokines would have the capacity to reduce the bone mineral density of patients with IH. However, the reason why monocytes were stimulated to increase cytokine production was not known.
In 1979, the existence of rats with spontaneous hypercalciuria (genetic hypercalciuric stone-forming, GHS) was described. By favoring breeding between them, a successive increase in calciuria was verified in the following generations. In search of the mechanism of hypercalciuria, Bushinsky and Favus observed that fourth generation rats had marked urinary calcium excretion due to increased intestinal calcium absorption, although calcitriol levels were normal. When rats were placed on a low-calcium diet, calciuria decreased, although it did not normalize, suggesting that increased intestinal calcium absorption was a mechanism that explained, at least in part, the hypercalciuria observed in these animals. In 1993, it was shown that in these rats there was an increase in the number of vitamin D receptors (VDR) in the intestine(10), which favored an increase in the functional capacity of the calcitriol-VDR complexes, thus explaining the increase in previously described intestinal calcium transport. Next, it was verified that, in these animals, there was an increased response of the VDRs to minimal doses of calcitriol, which implies that very high levels of calcitriol were not necessary to amplify their response and greatly increase calciuria. Indeed, Krieger et al. showed that this increased sensitivity to calcitriol was also expressed in the bones of these animals, inducing greater bone resorption(11), which seemed to show that bone also plays a role in the development of hypercalciuria. Later, it was found that in GHS rats there is also a defect in the renal tubular reabsorption of calcium.
In summary, hypercalciuric rats have many features in common with humans with IH, namely: normal calcium levels, intestinal calcium hyperabsorption, increased bone resorption, and defective renal tubular calcium reabsorption. In addition, their calcitriol levels are normal, as are in most patients with IH.
In 2004, Favus et al. demonstrated that peripheral monocytes from humans with IH have an increased number of VDR(12), that is, the same as previously observed in hypercalciuric rats(10).
Figure 3 shows the proposal for a pathophysiological scheme for IH, indicating that the cause of IH would be related to the existence of an increase in the number of VDRs, both in intestinal and bone cells (osteoclasts) as well as in peripheral monocytes.
Figura 3. Physiopathological diagram of idiopathic hypercalciuria (IH) which shows that the cause of IH would be related to the existence of an increase in the number of receptors for vitamin D, both in intestinal and bone cells (osteoclasts), as well as in peripheral monocytes. VDR: vitamin D receptor; CaSR: calcium sensitive receptor; IL-1: interleukin-1α; PGE2: prostaglandin E2; RANKL: nuclear factor kappaB (NF-κB). Elaborated by the authors. García-Vao Bel CM, García-Nieto VM, Teixeira Trindade AA. Hipercalciuria idiopática (Idiopathic hypercalciuria). In: Nefrología Pediátrica. Exeni R, García-Nieto V, Medeiros M, Santos F, eds. Oviedo: Ediciones de la Universidad de Oviedo; 2021. p. 999-1004.
Diagnosis and complementary tests
Calciuria determination in 24-hour urine in continent patients is the standard criteria for the diagnosis of hypercalciuria. The diagnosis is made if calciuria is greater than 4 mg/kg/day and is confirmed in a second determination or if a high value of the calcium/creatinine ratio is observed in an isolated urine sample (Table II). In a family environment of hypercalciuria and/or renal lithiasis, blood tests can be ignored or delayed; since, usually, they will be normal. However, to confirm that a patient with hypercalciuria has IH, the blood levels of calcium, sodium, potassium, chloride, phosphate, magnesium, and intact PTH must be normal, as well as the acid-base balance (Algorithm).
Renal ultrasound is mandatory to rule out the existence of lithiasis, microcalculi (concretions smaller than three millimeters in diameter without acoustic shadow), nephrocalcinosis, and associated malformations, since many renal malformations are accompanied by hypercalciuria.
If fractures have occurred or there is frequent bone pain, a bone densitometry would be indicated. It is necessary to be cautious before considering pharmacological treatment in the event of a loss of bone mass; since, in many cases, especially after puberty, there is a spontaneous improvement in bone mineral density(13).
Treatment
In our opinion, IH is not a disease but a “metabolic abnormality” or, better, a constitutive metabolic characteristic that is inherited, such as skin color, the number of fingers on the hand, or final height. In this sense, what patients with IH would inherit is the availability of possessing a greater number of VDRs in their cells than people with normal calciuria. Although they transmit the condition to their offspring, many people do not form stones or have reduced bone mass or remain asymptomatic for long periods of time(9). For this reason, it cannot be considered stricto sensu a disease, although in some cases it may predispose to the formation of kidney stones, the appearance of urinary tract infections and the development of osteopenia in the long term. This is a fundamental reason why the use of prolonged pharmacological treatment should be selected.
The normalization of calciuria should not be a universal objective in the treatment of these children, since it can be reduced spontaneously over the years and/or the formation of stones can be avoided with a diet rich in protective factors. In many cases, calciuria remains elevated despite correct dietary guidelines. Rather, the goal of dietary treatment should be to reduce the risk of crystallization, which is achieved by increasing urine volume and reducing the calcium/citrate ratio (see below). Raising awareness of the disease should be avoided as far as possible, so dietary recommendations should not be exhaustive. Ongoing physical exercise is very beneficial to improve bone mineral density.
At diagnosis, except for severe symptoms, dietary standards should be insisted on, in order to achieve an increase in the gain of protective factors against the formation of stones, through an increase in the intake of water, fruits (especially citrus fruits rich in citrate), vegetables (providing water and magnesium), oily fish (due to its richness in ω-3 fatty acids) and whole grains (due to their richness in phytate) (Table III).
There is also a commercialized phytate-based product (Broken®). It is sometimes difficult to get stone or prelithiasis children to drink enough water, because many of them have a permanently stimulated ability to concentrate. This fact is difficult to interpret except in those with palmoplantar hyperhidrosis; in these cases, the consumption of fruits and vegetables that are rich in water in their composition should be encouraged. Regarding drinking water, a/hypomineralized waters are not required. Avoiding hypermineralized (“hard water”) water should suffice.
To reduce the factors that promote stone formation, excess salt and protein consumption should be avoided (Table III).
Drug treatment should be reserved for two circumstances. In the first place, when there are marked clinical data such as: sustained dysuria, frequent macroscopic hematuria, or recurrent nephritic colic. Secondly, when calculi or nephrocalcinosis are observed on the ultrasound. Even in the presence of microcalculi, dietary treatment could be indicated, for at least one year, to observe progression. Four types of preparations are available, namely: potassium citrate, thiazides, bisphosphonates and the phytate-based products on the market.
Citrate salts (Acalka®, Shohl’s solution, Polycitra, Polycitra K) do not normalize calciuria unless there is a defect in acidification, but they form complexes with calcium and the formation of calcium oxalate and phosphate salts. The therapeutic target should be chosen to maintain a calcium/citrate ratio <0.33 mg/mg. In children, we have used a single nocturnal dose (“stones form at night”) with good results.
If citrate is not tolerated or there is no improvement, it is replaced by hydrochlorothiazide: 1.5-2.5 mg/kg in a single morning dose. Thiazides stimulate transcellular calcium transport in the distal convoluted tubule. It must be kept in mind that they can have side effects such as: hypokalemia, hypomagnesemia and increased plasma levels of glucose, urate, cholesterol and LDL. Its use should not be prolonged.
In cases of severe osteopenia, the antiresorptive effect of bisphosphonates has been tested in adults and in hypercalciuric adolescents with bone demineralization. In addition, calciuria is reduced.
We use the commercialized phytate-based products (Broken®) administered at night in cases where there is reasonable doubt about whether or not to start pharmacological treatment, as is the case of microcalculi, to observe the progression during a short term, or in the event that citrate-based preparations are not tolerated.
The concept of prelithiasis. The calcium/citrate ratio
The concept of prelithiasis, although it has not been generalized, includes patients with metabolic abnormalities that cause stones before they have symptoms of renal colic. It is basically a pediatric term and includes children with hypercalciuria and hypocitraturia, since the other causes of stones usually manifest with symptoms of kidney stones. The easiest way to screen for prelithiasis is the determination of the calcium/citrate ratio in the first urine of the day. Calcium/citrate ratio values greater than 0.33 indicate that urine is potentially lithogenic, regardless of age and time of urine collection.
Since the early 1980s, pediatricians have learned to identify children with metabolic abnormalities that cause stones in a phase of life in which they have not yet had time to form or develop them. This situation, almost specific to the pediatric age, has been called “prelithiasis”(3,14). The term has been somewhat contested, since not all people with metabolic abnormalities potentially causing stones form them in the long term. However, we believe that it is a descriptive and valid term.
Alexander Randall (1883-1951), the famous American urologist who described the plaques that bear his name, used the term “symptomatic silence” that could be equated in a certain way with that of prelithiasis. Thus, he wrote that: “A kidney stone is known clinically only after its growth has reached what we might call maturity. The fact that this growth has taken time is evident, as is the fact that during this time there has been a symptomatic silence”.
As indicated in the preceding lines, children with IH may present with symptoms or signs such as: gross or microscopic hematuria, sterile leukocyturia, dysuria, frequency, urinary urgency, urinary incontinence, nocturnal enuresis, cloudy urine, recurrent abdominal pain(4) or urinary infection(6,8,14).
The morphological expression of prelithiasis would coincide with the repeated vision of the same type of crystals in the urinary sediment and with the finding of mobile hyperechogenic particles in the bladder ultrasound that may be a transcript of red blood cells, leukocytes or crystals in the urine(15). We have verified that this crystalluria can be associated with hypercalciuria or hypocitraturia. The relationship between these last two metabolic anomalies is complex and a matter pending to be resolved; since, on occasions, they can coincide at the same time in the absence of a defect in renal acidification capacity. In addition, very frequently, it is observed that children with IH, as they approach adolescence, normalize urinary calcium excretion and concurrently show hypocitraturia(13). Sometimes, in adults who have passed stones and who are parents of children with IH, it is found that they are only carriers of hypocitraturia. It seems, therefore, as if both metabolic abnormalities had, in certain way, a link in common.
The diagnosis of prelithiasis condition is, therefore, another version of preventive medicine, since after its detection, dietary and/or pharmacological measures can be taken to prevent progression to lithiasic disease.
The easiest way to screen for prelithiasis is the determination of the calcium/citrate ratio in the first urine of the day. Urine is particularly lithogenic when there is an imbalance between the beneficial component (calcium) and the protective component (citrate). This imbalance is more evident at night, since together with the increase in urinary osmolality due to the lack of fluid intake during sleep, in many cases, an increase in calciuria due to a certain increase in bone resorption and a reduction of citraturia as a consequence of mild nocturnal physiological acidosis, which is a consequence of the formation of ketoacids given the lack of caloric intake(3,16). For this reason, the first urine of the day that collects the testimony of what happened at night must necessarily be studied independently. Calcium/citrate ratio values greater than 0.33 indicate that urine is potentially lithogenic, regardless of age and time of urine collection(17). Thus, in a study by our aforementioned group, the calcium/citrate ratio corresponding to urine at night was increased in 37.5 % of the samples and, on the other hand, the same ratio, in the first urine of the day was elevated in 80 % of the cases. This was the only parameter calculated that was also related to the existence of a family history of lithiasis(3). A particular situation may occur, preferably in adolescence, when citraturia may be reduced and also calciuria, with which the urine will not be lithogenic as it shows a normal calcium/citrate ratio.
Hypocitraturia
Hypocitraturia is frequently seen in patients with renal lithiasis. It can be observed in isolation or associated with hypercalciuria. It is a potent crystallization inhibitor. Sometimes it is not possible to know the cause of hypocitraturia, although metabolic acidosis, a diet with excess protein, cystic fibrosis, intestinal malabsorption and obesity must be ruled out.
Citrate acts as an inhibitor of calcium stone formation by forming a soluble complex, which decreases the availability of free ionic calcium necessary for crystallization of calcium oxalate or calcium phosphate. Citrate also acts as a direct inhibitor of the aggregation of calcium crystals and their growth(18); therefore, reduced urinary citrate may be an important cause of calcium lithiasis.
In general, metabolic acidosis is accompanied by hypocitraturia and alkalosis by hypercitraturia(19).
The frequency of hypercalciuria and, above all, hypocitraturia in cases of renal tubular acidosis is very high. Citrate is a very sensitive marker of metabolic acidosis(19). When the pH of the proximal tubular cell is reduced, the activity of the dicarboxylate cotransporter NADC1 (sodium-dependent dicarboxylate transporter 1) located in the apical membrane of the proximal tubule is stimulated, thereby passing more citrate into the cell. Citrate participates in the Krebs cycle in mitochondria, producing CO2 and H2O, a process in which three H+ ions are consumed, a reaction equivalent to generating bicarbonate.
Other causes of hypocitraturia, in addition to metabolic acidosis, include acetazolamide or thiazide therapy, potassium depletion, starvation, cystic fibrosis, intestinal malabsorption, and obesity. Excessive protein intake also favors its appearance due to the acid overload it causes. However, sometimes no known cause is detected (idiopathic hypocitraturia). In chronic renal failure, a reduction in citraturia may be indicative of an intracellular acidosis capable of initiating early alkalinizing treatment. As previously indicated, hypocitraturia associated with IH is observed with certain frequency, in the absence of an acidification defect.
We have observed in some cases that after the use of citrate, the ultrasonographic microcalculi may disappear. It should not be forgotten that in 1857, Spiller observed that citric acid has a special ability to keep calcium in solution. In the presence of citrate, calcium is not precipitated by carbonate, phosphate, or oxalate. When some of these precipitates have formed, they can be brought back into solution by additional citrate.
Role of the primary care pediatrician
An appropriate medical history, a correct physical examination, and basic renal function tests can lead the Primary Care pediatrician to a diagnosis of IH. This professional can proceed with subsequent control after recommending adequate dietary standards. The presence of morphological anomalies (ultrasound calculi) or suggestive symptoms would be an indication for referral of the patient to a Center specialized in Pediatric Nephrology.
As reflected in the article, IH is the most frequent cause of lithiasis, both in childhood and in adulthood, although the most common scenario is that it is asymptomatic or presents symptoms of prelithiasis (hematuria, micturition symptoms, abdominal pain…). In Primary Care it is important to suspect it in the face of the aforementioned symptoms, and perform the relevant tests for its diagnosis. Although 24-hour urine collection is standardized, it may be initially sufficient to determine the calcium/creatinine and calcium/citrate ratios in a first morning urine sample, the results of which must be confirmed in a subsequent sample.
Once the diagnosis has been made, it will be necessary to provide dietary measures, whether the patient is asymptomatic and has been a casual diagnosis, or if he presents associated symptoms. These indications will be essential to reduce symptoms, as well as to prevent the formation of renal lithiasis in the future.
Bearing in mind that IH is an entity whose cause is multifactorial, but in which genetics plays a fundamental role, one should not be obsessed with achieving normalization of calciuria. It will be essential, above anything else, to try to increase the levels of inhibitors of crystallization in the urine (citrate and magnesium), recommending foods rich in them, as well as in phytate (present in cereals and legumes), which is also a potent inhibitor of crystallization.
Unless complications arise, children with IH can be monitored by Primary Care pediatricians.
Conflict of interests
There is no conflict of interest in the preparation of the manuscript. Declaration of interests: none.
Bibliography
The asterisks reflect the interest of the article in the opinion of the authors.
1.*** Grases Freixedas F, Costa-Bauzá A. Mecanismos de la formación de los cálculos renales (Mechanisms of kidney stone formation). In: Nefrología Pediátrica. Exeni R, García-Nieto V, Medeiros M, Santos F, eds. Oviedo: Ediciones de la Universidad de Oviedo. 2021. p. 977-90.
2.** Lumbreras J, Rodrigo MD, Prieto RM, Costa-Bauzá A, Sanchis P, Espinosa N, et al. Comparing different morpho-compositional groups of kidney stones in a Pediatric Renal Lithiasis Registry. Pediatr Nephrol. 2021; 36: 3352.
3. García Nieto VM, Pérez Bastida XI, Salvador Cañibano M, García Rodríguez VE, Monge Zamorano M, Luis Yanes MI. Quantification of the risk of urinary calcium stone formation in the urine collected at 2 times of the day in a group of children studied to rule out prelithiasis. Nefrologia (Engl Ed). 2018; 38: 267-72.
4. Penido MG, Tavares MS. Beyond kidney stones: Why pediatricians should worry about hypercalciuria. World J Clin Pediatr. 2021; 10: 137-50.
5. Moore ES. Hypercalciuria in children. Hypercalciuria in children. Contr Nephrol. Basel: Karger. 1981; 28: 20-32.
6.** Cervera A, Corral MJ, Gómez Campdera FJ, De Lecea AM, Luque A, López Gómez JM. Idiopathic hypercalciuria in children. Classification, clinical manifestations and outcome. Acta Paediatr Scand. 1987; 76: 271-8.
7. Praga M, Martínez MA, Andrés A, Alegre R, Vara J, Morales E, et al. Association of thin basement membrane nephropathy with hypercalciuria, hyperuricosuria and nephrolithiasis. Kidney Int. 1998; 54: 915-20.
8. Sotoca Fernández J, O’Hagan M, Arango Sancho P, Luis Yanes MI, García Nieto V. A family history of renal lithiasis in children diagnosed of urinary tract infection by Escherichia coli. An Pediatr (Barc). 2018; 88: 204-8.
9.** García Nieto VM, Luis Yanes MI, Tejera Carreño P, Pérez Suárez G, Moraleda Mesa T. The idiopathic hypercalciuria reviewed. Metabolic abnormality or disease? Nefrologia (Engl Ed). 2019; 39: 592-602.
10.*** Li XQ, Tembe V, Horwitz GM, Bushinsky DA, Favus MJ. Increased intestinal vitamin D receptor in genetic hypercalciuric rats. A cause of intestinal calcium hyperabsorption. J Clin Invest. 1993; 91: 661-7.
11. Krieger NS, Stathopoulos VM, Bushinsky DA. Increased sensitivity to 1,25(OH)2D3 in bone from genetic hypercalciuric rats. Am J Physiol. 1996; 271: C130-5.
12.** Favus MJ, Karnauskas AJ, Parks JH, Coe FL. Peripheral blood monocyte vitamin D receptor levels are elevated in patients with idiopathic hypercalciuria. J Clin Endocrinol Metab. 2004; 89: 4937-43.
13.*** Pérez-Suárez G, Luis-Yanes MI, Martín-Fernández de Basoa MC, Sánchez-Almeida E, García-Nieto VM. Evolution of bone mineral density in patients with idiopathic hypercalciuria: a 20-year longitudinal study. Pediatr Nephrol. 2021; 36: 661-7.
14. Kamińska A, Jung A. Results of the treatment of pre-urolithiasis state in children with recurrent urinary tract infections. Pol Merkur Lekarski. 2000; 8: 209-10.
15. Luis Yanes MI, López Figueroa A, Arango Sancho P, Tejera Carreño P, Monge Zamorano M, Aracil Hernández D, et al. Ultrasonography findings of echogenic material in the bladder of a pediatric cohort. Pediatr Nephrol. 2018: 33: 1980.
16.** Mir C, Rodríguez A, Rodrigo D, Sáez-Torres C, Frontera G, Lumbreras J, et al. Analysis of urine composition from split 24-h samples: use of 12-h overnight samples to evaluate risk factors for calcium stones in healthy and stone-forming children. J Pediatr Urol. 2020; 16: 371.e1-e7.
17. Srivastava T, Winston MJ, Auron A, Alon US. Urine calcium/citrate ratio in children with hypercalciuric stones. Pediatr Res. 2009; 66: 85-90.
18. Tekin A, Tekgul S, Atsu N, Sahin A, Ozen H, Bakkaloglu M. A study of the etiology of idiopathic calcium urolithiasis in children: hypocitruria is the most important risk factor. J Urol. 2000; 164: 162-5.
19. Brennan S, Hering-Smith K, Hamm LL. Effect of pH on citrate reabsorption in the proximal convolute tubule. Am J Physiol. 1988; 255: F301-6.
20. González-Lamuño Leguina D. Hipercalciuria. Pediatr Integral. 2017; XXI: 529-40.
Recommended bibliography
– Grases Freixedas F, Costa-Bauzá A. Mecanismos de la formación de los cálculos renales (Mechanisms of kidney stone formation). In: Nefrología Pediátrica. Exeni R, García-Nieto V, Medeiros M, Santos F, eds. Oviedo: Ediciones de la Universidad de Oviedo. 2021. p. 977-90.
Chapter recently written by one of the most expert groups in lithiasis in our country. The complex mechanisms and causes involved in the formation of kidney stones are reviewed in a very educational way.
– Li XQ,Tembe V, Horwitz GM, Bushinsky DA, Favus MJ. Increased intestinal vitamin D receptor in genetic hypercalciuric rats. A cause of intestinal calcium hyperabsorption. J Clin Invest. 1993; 91: 661-7.
The study of hypercalciuric rats allowed us to suspect that they share mechanisms in their pathophysiology with human IH. The finding of an increase in the number of vitamin D receptors was essential in this regard.
– Pérez-Suárez G, Luis-Yanes MI, Martín-Fernández de Basoa MC, Sánchez-Almeida E, García-Nieto VM. Evolution of bone mineral density in patients with idiopathic hypercalciuria: a 20-year longitudinal study. Pediatric Nephrol. 2021; 36: 661-7.
This study has revealed for the first time that the urinary elimination of calcium in IH is not permanent and that, on occasions, it is replaced by hypocitraturia. This work has also revealed that the loss of bone mass observed in some patients with IH improves spontaneously in adolescence and young adults, especially in women.
| Clinical case |
|
A 7-year-old patient (weight: 23 kg, height: 118.5 cm) was referred because of a 12 day course of macroscopic hematuria at the end of urination, which was not accompanied by fever or dysuria. The family was initially assisted by their doctor, who had prescribed the administration of intramuscular penicillin and when the terminal hematuria did not subside, he was referred to us. Physical examination was normal except for a I/VI systolic murmur with functional characteristics in the mitral focus. In the personal history, it was noted that he had undergone adenoidectomy and tonsillectomy. His mother had suffered two renal colic. The macroscopic hematuria subsided two days after admission, remaining with persistent microhematuria (8-10 leukocytes per field, 20-25 red blood cells per field). The urine culture was negative and the full blood count was normal (Hb: 14 g/dL). General biochemistry was normal (creatinine: 0.5 mg/dl, uric acid: 3.5 mg/dl, calcium: 10 mg/dl), including proteinogram, immunoglobulins, and hepatitis B serology. Complement levels were C3: 97 mg/dl and C4: 16 mg/dl. The pharyngeal swab was negative. Renal ultrasound was normal. The volume of 24-hour urine delivered to the laboratory was 500 ml. In this sample, the creatinine concentration was 60 mg/dl and the calcium 17 mg/dl. In addition, magnesium: 6.2 mg/dl; uric acid: 47.6 mg/dl and citrate 300 mg/l.
|
 Hypercalciuria and hypocitraturia. The concept of prelithiasis in Pediatrics
Hypercalciuria and hypocitraturia. The concept of prelithiasis in Pediatrics